Abstract
We report the case of a 43-year-old male with both giant left atrial appendage (LAA) aneurysm and drug-refractory atrial fibrillation (AF). The patient was treated with percutaneous electrical isolation of cardiac arrhythmogenic substrate, and has been free of AF symptom over one year. Although the surgical resection of giant LAA aneurysm is mostly used to prevent systemic thromboembolism, we have performed follow-up of the giant LAA aneurysm using cardiac magnetic resonance (CMR) imaging and transesophageal echocardiography (TEE) after the successful catheter ablation of refractory AF. At one-year follow-up CMR, the giant LAA aneurysm showed remarkable enlargement as well as decreased contractility. Additionally, one-year follow-up TEE showed spontaneous echo contrast as an indicator of blood stasis in the giant LAA aneurysm. Those findings of giant LAA aneurysm suggest that the risk of thromboembolism may be high despite termination of AF.
A giant left atrial appendage (LAA) aneurysm is a very rare cardiac anomaly, and associated with the supraventricular arrhythmia including atrial fibrillation (AF) (123). Furthermore, the giant LAA aneurysm may be the main source of thrombosis in patients with AF (123). With technical developments of catheter ablation, the percutaneous electrical isolation of cardiac arrhythmogenic substrates has been considered as the effective strategy for management of drug-refractory AF (4). Additionally, Hof et al. (5) reported that a patient with AF and giant LAA aneurysm could be successfully treated by percutaneous pulmonary vein antrum isolation.
Echocardiography is the first line imaging technique to detect the giant LAA aneurysm (2). Especially transesophageal echocardiography (TEE) allows clear visualization of the LAA, and can be effective for the diagnosis of giant LAA aneurysm and thrombi. However, TEE may provide a relatively narrow field of view in the delineation of entire parts of giant LAA aneurysm (2). The role of cardiac magnetic resonance (CMR) has been emphasized in the comprehensive evaluation of cardiac anatomy, function, and tissue characteristics (6). In patients with AF, the CMR imaging has been actively applied to guide the catheter ablation of AF. Moreover, CMR data with isotropic voxel can be reformatted in the three-dimensional (3D) volumetric quantification of particular cardiac structure (7). To the best of our knowledge, the change of giant LAA aneurysm, treated with catheter ablation of AF has not been reported. Recently, we had the opportunity to review the giant LAA aneurysm treated with the catheter ablation of refractory AF using CMR imaging and TEE.
A 43-year-old man was referred to our institute for management of drug-refractory AF. The patient had no family history of structural heart disease or sudden death and no personal medical history. He had suffered from palpitation. Electrocardiogram (ECG) result was consisted with AF at the admission. TEE revealed the giant LAA aneurysm (> 5 cm in long diameter) but no intracardiac thrombus (Fig. 1). Then, he underwent CMR examination in evaluation of the giant LAA aneurysm using a 3-T magnetic resonance system (Achieva; Philips Medical Systems, Best, The Netherlands) with a 32-element phased-array cardiac coil. The CMR examination included 1) time resolved CMR angiography using 3D spoiled gradient-echo contrast-enhanced timing robust angiography (CENTRA) sequence, and 2) cine-CMR images using a steady-state free precession sequence. On the baseline CMR images (Fig. 1), the volume of giant LAA aneurysm was 158 cm3 (Fig. 1). Sequentially, percutaneous electrical isolation of cardiac arrhythmogenic substrates located at the pulmonary veins and giant LAA aneurysm was performed with the catheter ablation. He has sustained sinus rhythm without AF symptom over one year after the catheter ablation.
At the one-year follow-up, he underwent TEE and CMR imaging of the giant LAA aneurysm (Fig. 2). On the one-year follow-up CMR images, the volume of giant LAA aneurysm was 205 cm3. In addition, the giant LAA aneurysm showed remarkable enlargement as well as minimal change in its maximum dimension during cardiac cycle. It indicated the decreased contractile of giant LAA aneurysm. Furthermore, on the one-year follow-up TEE in sinus rhythm, the giant LAA aneurysm showed spontaneous echo contrast as an indicator of blood stasis and risk of thromboembolism. Eventually, the patient continued anticoagulation therapy, and we planned for surgical removal of the giant LAA aneurysm.
LAA aneurysm can be diagnosed if it is bigger than 3 cm on TEE (2). In particular, when the direct length from the LAA ostium to the LAA distal apex is longer than 5 cm, the LAA aneurysm can be considered as "giant" (2). The giant LAA aneurysm is a very rare cardiac anomaly from congenital dysplasia of LAA muscle pectinate (1). The LAA can be a source of AF triggering or a part of reentry, and it is usually associated with life threating systemic thromboembolism (5).
Surgical resection of the giant LAA aneurysm addresses potential intra-aneurysmal thrombi, and has been sufficient in eliminating AF (13). It is advised to perform this treatment even in asymptomatic patients to prevent AF and thromboembolic events (13). However, no large trials have been performed to evaluate the efficacy of surgical resection of the giant LAA in patients with AF. Mathur et al. (3) suggested that the triggers of AF may be located outside of the giant LAA aneurysm, and Cox-Maze III procedure for effective surgical cure of AF should be performed in addition of surgical resection. Depending on the pathophysiology of AF, eventually, the surgical operation may be complex in patients who have both giant LAA aneurysm and AF.
Catheter ablation is a good therapeutic option, when at least one anti-arrhythmic drug has failed (4). Hof et al. (5) reported that a patient with both giant LAA aneurysm and AF could be successfully treated by catheter ablation. When focusing on the morphometric remodeling of LAA, the size of LAA can be reversed after successful ablation of AF (8). Progressive dilatation of the LAA may be associated with an impaired LAA systolic function and thrombus formation (8). Therefore, decreased size of the LAA after the catheter ablation may theoretically indicate an improvement in the LAA function and decrease the stroke risk (8). In our case, the AF which developed from giant LAA aneurysm was terminated with the catheter ablation. However, the giant LAA aneurysm itself showed paradoxical response after catheter ablation.
Cardiac imaging plays a key role in patient selection and prediction of safety and efficacy for catheter ablation of AF (6). Non-invasive imaging modalities including TEE and CMR are combined for the assessment of underlying structural heart disease, exclusion of intra-cardiac thrombus, identification of cardiac anatomy and function (6). In our case, CMR imaging provided detail visualization and 3D volumetric quantification of giant LAA aneurysm. We believe that the volume assessment of LAA may be useful especially in diagnosis of the progressive dilation of giant LAA aneurysm.
In summary, we described the progressive dilatation and functional impairment of giant LAA aneurysm as the paradoxical response after the successful catheter ablation of AF using CMR imaging. We believe that those CMR findings can help understand the hemodynamic risks of thromboembolism in the giant LAA aneurysm in normal sinus rhythm.
Figures and Tables
Fig. 1
Baseline cardiac magnetic resonance (CMR) and transesophageal echocardiography (TEE) of the giant left atrial appendage (LAA) aneurysm before the catheter ablation of atrial fibrillation (AF). CMR angiography (a) describes entire appearance of LAA aneurysm and left ventricle (LV). On the CMR angiography, the segmentation of LAA aneurysm (green color) allows the volume assessment of giant LAA aneurysm as 158 cm3 (b, c). On cine-CMR images (d, e), the maximum dimensions of LAA aneurysm at left ventricular systole and diastole are aneurysm shows 34.75 mm and 24.01 mm, respectively. TEE image (f) shows no thrombus and spontaneous echo contrast in the giant LAA aneurysm.
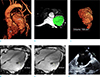
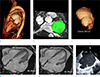
Fig. 2
One year follow-up cardiac magnetic resonance (CMR) and transesophageal echocardiography (TEE) of the giant left atrial appendage (LAA) aneurysm after catheter ablation of atrial fibrillation (AF). CMR angiography (a) describes entire appearance of LAA aneurysm covering the left ventricle. On the CMR angiography, the segmentation of LAA aneurysm (green color) provides the volume assessment of giant LAA aneurysm as 205 cm3 (b, c). On the cine-CMR images (d, e), the maximum dimensions of LAA aneurysm at left ventricular systole and diastole are aneurysm shows 46.34 mm and 42.76 mm, respectively. Furthermore, TEE image (f) shows spontaneous echo contrast related to blood stasis in the giant LAA aneurysm.
References
1. Conradi G, Deetjen A, Mollmann S, Hamm CW, Dill T. Symptomatic atrial fibrillation as the first symptom of a giant left atrial appendage aneurysm. Clin Res Cardiol. 2006; 95:614–616.
2. Ulucam M, Muderrisoglu H, Sezgin A. Giant left atrial appendage aneurysm: the third ventricle. Int J Cardiovasc Imaging. 2005; 21:225–230.
3. Mathur A, Zehr KJ, Sinak LJ, Rea RF. Left atrial appendage aneurysm. Ann Thorac Surg. 2005; 79:1392–1393.
4. Bhargava M, Di Biase L, Mohanty P, et al. Impact of type of atrial fibrillation and repeat catheter ablation on long-term freedom from atrial fibrillation: results from a multicenter study. Heart Rhythm. 2009; 6:1403–1412.
5. Hof IE, Wildbergh TX, van Driel VJ, et al. Atrial fibrillation with a giant left atrial appendage can be successfully treated with pulmonary vein antrum isolation. Neth Heart J. 2012; 20:179–181.
6. Bhagirath P, van der Graaf AW, Karim R, et al. Multimodality imaging for patient evaluation and guidance of catheter ablation for atrial fibrillation - current status and future perspective. Int J Cardiol. 2014; 175:400–408.
7. Hwang SH, Oh YW, Lee DI, Shim J, Park SW, Kim YH. Evaluation of quantification methods for left arial late gadolinium enhancement based on different references in patients with atrial fibrillation. Int J Cardiovasc Imaging. 2015; 31:Suppl 1. 91–101.
8. Chang SH, Tsao HM, Wu MH, et al. Morphological changes of the left atrial appendage after catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2007; 18:47–52.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download