Abstract
The spine is the most common location for skeletal metastases, and the incidence of spinal metastasis shows an increasing tendency. Because metastatic spinal tumors progress from an anterior element to a posterior element resulting in continuing destruction of the pedicles, epidural extension and involvement of neural structures of the metastatic tumor are eventually visible. Therefore, it is clinically significant for radiologists to understand the pathophysiology of spinal metastasis and to assess the involvement of neural structures and the disintegration of spinal instability related to the pathophysiology. As MRI is also the best imaging modality for diagnosing spinal metastasis, radiologists should accurately assess spinal metastasis and provide practical information to physicians. Therefore, we will describe some analysis points focusing on the understanding of pathophysiology of spinal metastasis and the next step toward a more extensive clinical approach using MR imaging.
The spine is the most common site for skeletal metastases, and the incidence of spinal metastasis tends to increase due to the increase in older populations and improvements in medical treatment, such as chemotherapy and radiotherapy (12). However, previous reports have focused on tumor detection and the differential diagnosis with other diseases and it was insufficient to provide physicians with practical information for the treatment of spinal metastasis.
Spinal metastasis results in neural compression and spinal column instability that can be cause significant pain, neurologic complications or both (3). These clinical representations of spinal metastasis seem to be correlated with the pathophysiology of the spinal metastasis described in the past (4). Therefore, we propose two learning objectives in our study. The first is to understand the pathophysiology of the spinal metastasis resulting in clinical symptoms and complications. The second is to evaluate the MR examination while focusing on some analysis points providing practical and useful information regarding the next step for a further developed clinical approach.
In the initial stage of spinal metastasis, the metastatic tumor more often occurs in the vertebral body than in posterior element due to the high vascular red marrow of the vertebral body. At this time, cancer patients complain primarily of pain. In the advanced stage, as the spinal metastatic tumor grows from the anterior to the posterior element, successive destruction of the pedicles and spinal epidural extension of the metastatic tumor occur (Fig. 1). At this time, cancer patients complain of both pain and neurologic complications resulting from neural compression and spinal instability (56). And depending on the clinical situation, patients may require surgical intervention or radiation therapy (7). Therefore, it is very valuable for radiologists to understand this pathophysiology of spinal metastasis and to evaluate the neural involvement of the neural structure and loss of spinal stability related to the pathophysiology.
Spinal metastasis can occur in three regions including the extradural, intradural extramedullary, and intramedullary regions (4). As most spinal metastases (more than 98%) occur in an extradural location, anatomical classification of the extradural location is very important (8). Anatomical classification can be useful for recognizing a patient's overall tumor load and deciding which type of clinical management to perform. Tomita et al. (9) proposed a classification (Fig. 2) composed of seven categories according to whether the metastasis is contained within the spinal bones (intracompartmental, Fig. 3), out of the bones (extracompartmental, Fig. 4) or with multiple vertebral involvement (Fig. 5). Though there are various anatomical classification systems, this is a simple classification which is easy to remember and to apply and which shows the natural stages of metastatic tumor progression from involvement of the vertebral body to the pedicles and posterior elements, to extradural and paravertebral space, and to adjacent vertebrae (1).
Neural compression caused by a metastatic tumor of the spine can result in significant pain and serious neurologic consequences. Patients may present with radicular abnormalities, myelopathic abnormalities or a combination of both (3610). As much as 10% of cancer patients can show symptomatic spinal metastasis at their initial presentation, and pain is the most common symptom of spinal metastasis (2). Pain is classified as three, classic pain syndromes, i.e. local, mechanical, and radicular pain. Local pain originates from the region or segment of the spine affected by the metastatic tumor. Mechanical pain is accelerated with movement of affected the spinal segment. Radicular pain can occur due to irritation, compression or invasion of the nerve root by epidural extension of the metastatic tumor (3) (Fig. 4). Spinal cord compression can also cause pain (11) (Fig. 6). Among the patients showing spinal cord compression, 90% present with pain and 47% present with neurologic symptoms. The second most common finding in patients showing neural compression cause by a metastatic spinal tumor is motor dysfunction. Especially in patients with metastatic epidural spinal cord compression, approximately 60-85% of such patients may present with motor weakness at the time of their diagnosis (4). Finally, neurologic complications include sensory deficit and autonomic dysfunction (6). Therefore, the accurate information regarding the neural compression caused by epidural metastasis and spinal cord compression and that can be obtained from MR imaging is very useful in assessing a cancer patient's symptoms.
For patients with spinal metastasis, spinal instability is a significant factor upon which physicians plan a treatment (12). The loss of spinal instability can result in movemen-trelated pain, progressive deformity and neural compromise under the physiological load (13). Tumor-related pathologic fracture can also not only cause pain, but can cause neurologic complications due to the loss of spinal integrity (1014) (Fig. 7). Because pathologic metastatic fractures of the spine can cause a debilitated state due to the pain and motor weakness, it is worthwhile for radiologists to assess degree and complication of spinal metastatic fracture detected by MR imaging (15). However, as previous reports did not provide an evidence-based guideline for the evaluation and treatment of spinal instability, the diagnosis of spinal instability has been difficult. In response, the Spine Oncology Study Group (SOSG) developed the Spinal Instability Neoplastic Scale (SINS) by collecting literatures as well as the opinions of clinical experts (Table 1). SINS determines the score of spinal instability by adding together six, radiographic and clinical components. The radiographic components include the radiographic spinal alignment, vertebral body collapse and posterolateral involvement of spinal elements (9). Fisher et al. (16) recommended SINS as a reliable tool for radiologists evaluating spinal instability, and it is possible to accurately discriminate between stable and potentially unstable or unstable lesions using this classification system. Therefore, radiologists need to become interested in using this classification system when evaluating spinal MR exams.
Leptomeningeal metastasis (Fig. 8): Metastatic spread of tumor cells to the leptomeninges of the central nervous system (CNS) is an increasingly common complication of cancer, resulting in significant neurologic disability and early death. Malignant cells are disseminated within the cerebrospinal fluid and replicate at various sites within the CNS. Cancer cells that penetrate into the cerebrospinal fluid (CSF) can form secondary deposits in the leptomeninges throughout the neuroaxis (1718).
Radiotherapy (RT) is the most common initial treatment used by oncologists. The benefits of RT include effective pain control and avoidance of systemic complications that may occur when using chemotherapy. RT is also relatively easy to perform (2). In the past, the role of surgery for metastatic spinal tumors has been undervalued. However, surgery can improve the mechanical stability, and cord compression and reduce the amount of pain (21). Chemotherapy is seldom considered as a choice for treatment of metastatic tumor of the spine because of its systemic complication and extended time to pain relief. However, successful chemotherapy can help to shrink the tumor burden and to decrease pain (222). Finally, epidural steroid injection, vertebroplasty or kyphoplasty are useful options for spinal metastases and metastatic pathologic fractures of the spine in order to control back pain (23).
In conclusion, it is important for radiologists to accurately understand the pathophysiology and to practically evaluate MR imaging. Analysis in MR imaging of spinal metastasis described in this article, such as the anatomical location, neural compression, and spinal instability, help physicians to choose a better treatment plan.
Figures and Tables
Fig. 1
Tumor in the vertebral body: The tumor is anterior to the spinal cord and grows posteriorly to compress the spinal cord (6). Reprinted with permission from (6): Cole JS, Patchell RA. Metastatic epidural spinal cord compression. Lancet Neurol 2008;7:459-466.
Fig. 2
Schematic diagram of the surgical classification of spinal tumors, from Choi et al. (1). Reprinted with permission from: Choi D, Crockard A, Bunger C, et al. Review of metastatic spine tumour classification and indications for surgery: the consensus statement of the Global Spine Tumour Study Group. Eur Spine J 2010;19:215-222.
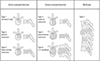
Fig. 3
Intracompartmental bone metastasis: 68-year-old woman consulting for breast cancer. Sagittal gadolinium-enhanced T1-weighted (TR/TE; 550/7) image with fat saturation (a) and axial gadolinium-enhanced T1-weighted (TR/TE; 455/10) image with fat saturation (b) show C7 metastasis contained within vertebral body.
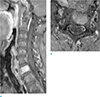
Fig. 4
Extracompartmental bone metastasis: 58-year-old man consulting for hepatocellular carcinoma. Sagittal gadolinium-enhanced T1-weighted (TR/TE; 711/11) image with fat saturation (a), axial T2-weighted (TR/TE; 5063/89) image (b) and axial gadolinium-enhanced T1-weighted (TR/TE; 741/11) image with fat saturation (c) show T7 metastasis out of the bones of T7 extending to epidural and a paravertebral space.
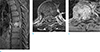
Fig. 5
Metastases in multiple vertebral levels: 42-year-old woman visiting for sigmoid colon cancer and both inguinal pain. Sagittal T1-weighted (TR/TE; 496/10) image (a), sagittal T2-weighted (TR/TE; 3832/100) image (b) and sagittal gadolinium-enhanced T1-weighted (TR/TE; 460/10) image with fat saturation (c) show multiple vertebral involvement of bone metastasis in L1-L5 and bodies of S1 and S2.
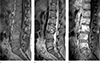
Fig. 6
Neural compression: 59-year-old man with esophageal cancer visited for weakness of both lower extremities. Sagittal T1-weighted (TR/TE; 661/10) image (a), sagittal T2-weighted (TR/TE; 2347/120) image (b), sagittal gadolinium-enhanced T1-weighted (TR/TE; 557/8) image with fat saturation (c), axial gadolinium-enhanced T1-weighted (TR/TE; 483/10) image with fat saturation (d), axial T2-weighted (TR/TE; 3262/115) image (e) show bone metastasis in T1 and T2 associated with pathologic compression fracture and epidural extension (empty arrows) of the metastatic tumor resulting in compromise of spinal canal and compression of spinal cord. And sagittal and axial T2-weighted images also show high signal change in spinal cord of C7-T4 level suggesting compressive myelopathy (arrows).
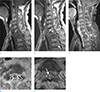
Fig. 7
Spinal instability: 51-year-old woman with breast cancer visiting for detection of metastasis in T7, but she did not complain of severe pain. Sagittal T2-weighted (TR/TE; 3500/100) image (a) shows bone metastasis and pathologic compression fracture without spinal cord compression. She treated by palliative radiation therapy for T7. However, two years later, in follow-up study, sagittal T2-weighted (TR/TE; 3500/120) image (b) shows more decreased height of T7 and compression of spinal cord in T7 level.
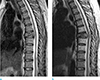
Fig. 8
Special cases: Leptomeingeal metastasis: 43-year-old woman with breast cancer complained of weakness and pain in both lower extremities. Sagittal T1-weighted (TR/TE; 661/10) image of C-T spine (a), sagittal T2-weighted (TR/TE; 2239/120) image of C-T spine (b) and sagittal gadolinium-enhanced T1-weighted (TR/TE; 448/10) image with fat saturation image of L-spine (c) show multiple bone metastases in the whole spine, and thick enhancement of leptomeninges from L5 to S2 suggesting leptomeningeal metastasis (arrows). Leptomeningeal metastasis is proven by CSF study.
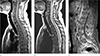
Table 1
The Spinal Instability Neoplastic Scale (SINS) Classification According to Fisher et al. (16)

The scores for the six radiographic and clinical components were added together to yield a total SINS score ranging from 0 to 18. Reprinted with permission from (16): Fisher CG, Versteeg AL, Schouten R, et al. Reliability of the spinal instability neoplastic scale among radiologists: an assessment of instability secondary to spinal metastases. AJR Am J Roentgenol 2014;203:869-874.
1Pain improvement with recumbency, pain with movement or loading of spine, or both.
2Facet, pedicle, or costovertebral joint fracture or replacement with tumor.
References
1. Choi D, Crockard A, Bunger C, et al. Review of metastatic spine tumour classification and indications for surgery: the consensus statement of the Global Spine Tumour Study Group. Eur Spine J. 2010; 19:215–222.
2. Harel R, Angelov L. Spine metastases: current treatments and future directions. Eur J Cancer. 2010; 46:2696–2707.
3. Sciubba DM, Gokaslan ZL. Diagnosis and management of metastatic spine disease. Surg Oncol. 2006; 15:141–151.
4. Ecker RD, Endo T, Wetjen NM, Krauss WE. Diagnosis and treatment of vertebral column metastases. Mayo Clin Proc. 2005; 80:1177–1186.
5. Mut M, Schiff D, Shaffrey ME. Metastasis to nervous system: spinal epidural and intramedullary metastases. J Neurooncol. 2005; 75:43–56.
6. Cole JS, Patchell RA. Metastatic epidural spinal cord compression. Lancet Neurol. 2008; 7:459–466.
7. Dunning EC, Butler JS, Morris S. Complications in the management of metastatic spinal disease. World J Orthop. 2012; 3:114–121.
8. Schick U, Marquardt G, Lorenz R. Intradural and extradural spinal metastases. Neurosurg Rev. 2001; 24:1–5. discussion 6-7
9. Tomita K, Kawahara N, Kobayashi T, Yoshida A, Murakami H, Akamaru T. Surgical strategy for spinal metastases. Spine (Phila Pa 1976). 2001; 26:298–306.
10. Galasko CS, Norris HE, Crank S. Spinal instability secondary to metastatic cancer. J Bone Joint Surg Am. 2000; 82:570–594.
11. Helweg-Larsen S, Sorensen PS. Symptoms and signs in metastatic spinal cord compression: a study of progression from first symptom until diagnosis in 153 patients. Eur J Cancer. 1994; 30A:396–398.
12. Fisher CG, DiPaola CP, Ryken TC, et al. A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine (Phila Pa 1976). 2010; 35:E1221–E1229.
13. Crim JR, Bassett LW, Gold RH, et al. Spinal neuroarthropathy after traumatic paraplegia. AJNR Am J Neuroradiol. 1988; 9:359–362.
14. Laufer I, Rubin DG, Lis E, et al. The NOMS framework: approach to the treatment of spinal metastatic tumors. Oncologist. 2013; 18:744–751.
15. Lee CS, Jung CH. Metastatic spinal tumor. Asian Spine J. 2012; 6:71–87.
16. Fisher CG, Versteeg AL, Schouten R, et al. Reliability of the spinal instability neoplastic scale among radiologists: an assessment of instability secondary to spinal metastases. AJR Am J Roentgenol. 2014; 203:869–874.
17. Chamberlain MC. Neoplastic meningitis. Neurologist. 2006; 12:179–187.
18. Shapiro WR, Johanson CE, Boogerd W. Treatment modalities for leptomeningeal metastases. Semin Oncol. 2009; 36:S46–S54.
19. Mostardi PM, Diehn FE, Rykken JB, et al. Intramedullary spinal cord metastases: visibility on PET and correlation with MRI features. AJNR Am J Neuroradiol. 2014; 35:196–201.
20. Lee SS, Kim MK, Sym SJ, et al. Intramedullary spinal cord metastases: a single-institution experience. J Neurooncol. 2007; 84:85–89.
21. Eastley N, Newey M, Ashford RU. Skeletal metastases - the role of the orthopaedic and spinal surgeon. Surg Oncol. 2012; 21:216–222.
22. Love RR, Leventhal H, Easterling DV, Nerenz DR. Side effects and emotional distress during cancer chemotherapy. Cancer. 1989; 63:604–612.
23. Cho JH, Ha JK, Hwang CJ, Lee DH, Lee CS. Patterns of treatment for metastatic pathological fractures of the spine: the efficacy of each treatment modality. Clin Orthop Surg. 2015; 7:476–482.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download