Abstract
Adenosarcoma of the uterus is a rare biphasic tumor containing benign glandular epithelial and malignant mesenchymal components. The tumor has been reported to be associated with antiestrogen therapy, particularly tamoxifen, but there have been a few case reports with MRI. We present two cases of MRI findings of uterine adenosarcoma after antiestrogen therapy, tamoxifen and toremifene in breast cancer patients. The tumor presents as a large polypoid mass occupying the endometrial cavity, and may protrude into the vagina. On MRI, the tumor typically shows solid components with scattered small cysts and heterogeneous enhancement. These findings are not significantly different from conventional adenosarcoma.
Adenosarcoma of the uterus is a mixed epithelial-mesenchymal tumor containing benign glandular epithelial component with sarcomatous mesenchymal stroma. This rare tumor accounts for only 8% of all uterine sarcomas (1). It has been suggested that some cases of the uterine adenosarcoma may be associated with hyperestrinism due to the administration of tamoxifen and prior radiation exposure (1, 2, 3).
Tamoxifen is an antiestrogen drug which is widely used as adjuvant therapy for breast cancer. However, it has been associated with proliferative abnormalities of the endometrium including endometrial hyperplasia, endometrial polyps, and cancer (2). Toremifene, a synthetic analogue of tamoxifen, also has been thought to be associated with endometrial cancer (7).
To our knowledge, there have been only a few case reports with documented MRI of uterine adenosarcoma including two radiologic reports of tamoxifen related adenosarcoma (2, 3, 4, 5, 6). Here, we report two cases of uterine adenosarcoma with regard to MRI features in patients receiving antiestrogen therapy for breast cancer.
A 69-year-old woman complained of watery vaginal discharge. She had a history of left breast-conserving surgery for invasive ductal carcinoma 8 years ago and had taken 40 mg of toremifene daily for 63 months. The patient underwent transvaginal sonography, which revealed 4×3.8 cm heterogeneous mass in endometrial cavity. MRI was performed using 3T unit (Trio-Tim; Siemens, Erlangen, Germany). The protocol included turbo spin-echo T1-weighted axial scans (Repetition Time (TR)/ Echo Time (TE) 538/11) and turbo spin-echo T2-weighted axial, oblique axial, and coronal scans (4000/113). After IV administration of gadolinium contrast agent, turbo spin-echo T1-weighted axial scans with/without fat suppression, turbo spin-echo T1-weighted coronal scans were also obtained.
MRI revealed an enlarged uterus with thin myometrium. The endometrial cavity was distended by a heterogeneous mass measuring 4.8×4.1×5.2 cm. The mass was predominantly solid with high signal intensity on T2-weighted image and intermediate signal intensity on T1-weighted image compared to the myometrium and had scattered small cystic portions. After gadolinium administration, the solid components showed avid enhancement. The tumor seemed to invade the myometrium at right anterior aspect of uterus. However there was neither lymphadenopathy nor pelvic ascites (Fig. 1a-c).
Hysteroscopy showed a large white, smooth surfaced tumor occupying the endometrial cavity, and she underwent hysteroscopic mass removal. Microscopically, the tumor was composed of glandular and stromal components. The glandular structures showed imparting a leaf-like appearance and were lined by cuboidal cells. The stroma was highly cellular and revealed high-grade cytologic atypia with frequent mitosis. The pathological diagnosis was adenosarcoma (Fig. 1d). The patient subsequently underwent hysterectomy with bilateral oophorectomy and pelvic lymph node dissection. Residual tumor was presented in endometrium. There was neither myometrial invasion nor lymph node metastasis at that time. But, three years following surgery, multiple nodules were newly observed in both upper lungs, and a diagnosis of metastatic adenosarcoma was made after pulmonary wedge resection.
A 47-year-old woman was referred to our hospital with vaginal spotting. She had a history of left mastectomy for invasive ductal carcinoma and had been treated with 20 mg of tamoxifen daily for 34 months. Pelvic examination revealed an enlarged uterus and a large mass protruding to the vagina (Fig. 2a). The patient underwent transvaginal sonography, which revealed a heterogeneous echogenicity of enlarged uterus and several hypoechoic masses within myometrium.
MRI was performed using 3T unit (Achieva; Philips Medical Systems, Amsterdam, Netherlands). The protocol included turbo spin-echo T1-weighted axial scans (TR/TE 550/10), turbo spin-echo T2-weighted axial, oblique axial, and coronal scans (4471/90), diffusion weighted image (DWI)(b=1000 s/mm2) and apparent diffusion coefficient (ADC) maps. After IV administration of gadolinium contrast agent, turbo spin-echo T1-weighted axial scans with/without fat suppression, turbo spin-echo T1-weighted coronal scans were also obtained.
MRI revealed an enlarged uterus and diffusely thickened junctional zone with ill-defined contours and embedded tiny T2 high signal intensity lesions, suggesting uterine adenomyosis. The endometrial cavity was occupied by a heterogeneous polypoid mass measuring 5.2×3.7×14 cm. The mass was attached to the uterine fundus by a stalk and protruded to the vagina throughout the cervical os. The mass was composed of predominantly solid portion with high signal intensity on T2-weighted image and intermediate to slightly high signal intensity on T1-weighted image compared to the myometrium. Tiny cystic areas and T1 high signal intensity areas presenting hemorrhagic foci are scattered within the mass. After gadolinium administration, the solid components showed diffuse and strong enhancement. There was no restricted diffusion on DWI and ADC map (Fig. 2b-f). The mass was considered as a benign uterine tumor such as adenomyoma or pedunculated endometrial polyp, rather than a malignant uterine tumor.
The patients underwent hysterectomy with bilateral oophorectomy. A large whitish gray mass, which was prolapsed through the external cervical os, was observed (Fig. 2g). Microscopic examination showed a neoplasm composed of glandular and stromal components. The glandular structures were lined by endometrioid or ciliated eosinophilic cuboidal cells and cystically dilated with intracystic papillary growth. The stroma was endometrial stromal type with collar-pattern and showed cellular atypia. It was limited to the endometrium with no myometrial invasion, and mitosis was rarely seen (2/10 HPFs). A diagnosis of adenosarcoma was made.
Tamoxifen has been used for both preventive and therapeutic treatment of breast cancer for more than 40 years. This drug acts as an antagonist of estrogen receptor in breast tissue. However, in endometrium, it behaves as an agonist, leading to proliferative complication of the endometrium such as post-menopausal endometriosis, endometrial polyps, endometrial hyperplasia and endometrial malignancy (2). Though endometrial adenocarcinoma is the most common malignancy associated with tamoxifen therapy, mesenchymal and mixed epithelial-mesenchymal malignancies such as leiomyosarcoma, endometrial stromal sarcoma, carcinosarcoma and adenosarcoma have rarely been described. Growing concern about the significant side effects of tamoxifen has driven the development of analogues including toremifene. However, a recent meta-analysis revealed no difference in number of endometrial cancers between toremifene and tamoxifen (7).
Adenosarcoma is a one of the mixed epithelial-mesenchymal tumors of the uterus, consisting of a benign glandular epithelial component with sarcomatous mesenchymal stroma. This tumor may be considered as an indeterminate state between benign adenofibroma and highly malignant carcinosarcoma (1, 2). Adenosarcoma accounts for 8% of all uterine sarcomas. The most common symptom is abnormal vaginal bleeding, and the mean age is 58 years (1). On gross pathologic examination, the tumor presents as a well demarcated large polypoid mass containing necrotic areas. They are usually solitary lesion arising from the fundus and occupying the endometrial cavity, occasionally protruding from cervix. Thus, they resemble a large endometrial polyp or submucosal myoma (2, 3).
On microscopic examination, the stromal component of adenosarcoma may include uterine elements (homologous) or elements not normally found in the uterus (heterologous). This sarcomatous component is generally a low-grade homologous sarcoma and has low malignant potential. The recurrence usually occurs in the pelvis, and the hematogenous metastasis is extremely rare. Pathological features predict-ing recurrence or metastasis are the presence of extrauterine spread, myometrial invasion and sarcomatous overgrowth characterized by sarcomatous component occupying at least 25% of total tumor (3, 4, 6, 8).
Total hysterectomy with bilateral salpingo-oophorectomy, followed by pelvic radiation therapy is a standard treatment. The prognosis of uterine adenosarcoma is very poor with only 10 to 25% 5-year survival rate (3).
Only a few cases have been reported MR features of uterine adenosarcoma (2, 3, 4, 5, 6). The adenosarcoma usually presents with enlarged uterus and a thin myometrium. The endometrial cavity is filled by a large polypoid mass that extend to the cervical os. The mass has solid components with iso to low signal intensity on T1-weighted image and high signal intensity on T2-weighted image compared to the myometrium and cystic components, reflecting glandular epithelial components (3, 6).
Differentiation of uterine adenosarcoma from benign tumor such as endometrial polyp, submucosal myoma or adenofibroma can be difficult. Endometrial polyp is frequently identified as focal mass within the endometrial cavity. Cystic spaces corresponding to dilated glands may be seen within the polyp. Submucosal myoma usually appears homogeneously hypointense on T2-weighted image relative to the myometrium or heterogeneously hyperintense when degeneration is present (9). Adenofibroma can be shown as homogeneously enhancing solid mass or small polypoid cystic masses in endometrial cavity (4). Carcinosarcoma, most aggressive end of the mixed epithelial-mesenchymal neoplasm spectrum, generally presents as a large broad based bulky polypoid mass. In contrast to adenosarcoma, carcinosarcoma may show greater heterogeneity. Extrauterine disease is seen in up to 30% of patients at initial presentation (10).
As we know, there have been only two MR imaging reports of uterine adenosarcoma that associated with tamoxifen therapy (2, 5). The first report by Chourmouzi et al. (2), described a heterogeneous mass with many well-defined cystic spaces and subacute hemorrhage. Lattice like septal enhancement was similar to that noted in endometrial polyps associated with tamoxifen treatment. Another report by Soh et al. (5) described heterogeneous polypoid mass with multiple cystic areas. The enhancement pattern was more heterogeneous rather than lattice like. The features of a fibrous vessel-containing pedicle entering the mass ("pedicle sign") and myometrial invasion suggested the adenosarcoma rather than a polyp (5). However, there was no significant difference in MR imaging findings between tamoxifen associated and non-tamoxifen associated adenosarcoma.
In our cases, the enhancement pattern was similar to the second report by Soh et al. However, there was no presence of a vascular pedicle entering the adenosarcoma in our cases. Diffusely thickened and ill-defined junctional zone in our case 2 was not usual finding in both tamoxifen associated and non-tamoxifen associated adenosarcoma. All previous reports of uterine adenosarcoma presented thin or slightly thin myometrium. No diffusion restriction in our case may be due to relatively lower cellularity and malignant potential in case 2.
In conclusion, we reported the MR imaging findings of two cases of uterine adenosarcoma associated with antiestrogen treatment in breast cancer patients. The patients undergoing antiestrogen therapy have a high risk for uterine adenosarcoma. The tumor usually presents as a heterogeneous polypoid endometrial mass with small cystic lesions on MRI and can mimic benign tumor such as endometrial polyp, submucosal myoma or adenofibroma. Thus, the possibility of uterine adenosarcoma should be kept in mind in patient undergoing long-term antiestrogen therapy even though imaging findings suggest the presence of a benign looking mass in the endometrial cavity.
Figures and Tables
Fig. 1
Case 1. A 69-year-old woman with uterine adenosarcoma.
Sagittal turbo spine-echo T2-weighted image (TR/TE, 4000/113) (a) shows the uterine cavity enlarged by a heterogeneous mass which is high signal intensity compared to the myometrium and have well delineated cystic spaces. On axial turbo spin-echo T1-weighted image (TR/TE 538/11) (b), the endometrial mass have intermediate signal intensity. Gadolinium-enhanced turbo spin-echo T1-weighted coronal image (c) shows strong enhancement of the solid components within the tumor. The tumor seemed to invade the myometrium at right side of uterus (arrow). Photomicrograph (× 40) (d) reveals the cellular stroma protruding into a dilated gland lumen. The gland is elongated and compressed, imparting a leaf-like appearance. The stromal cells show high-grade cytologic atypia with mitotic figures (not shown).
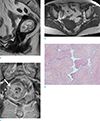
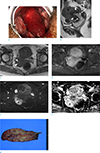
Fig. 2
Case 2. A 47-year-old woman with uterine adenosarcoma.
Pelvic examination (a) reveals a large polypoid mass protruding to the vagina. Sagittal turbo spine-echo T2-weighted image (TR/TE, 4471/90) (b) shows a heterogeneous polypoid mass occupying the endometrial cavity and protruding from the uterine cervix to the vaginal cavity. The mass shows high signal intensity compared to the myometrium and has tiny hyperintense cystic areas within the mass (arrows). Enlarged uterus with thickened junctional zone is also noted (thick arrows). On axial turbo spin-echo T1-weighted image (TR/TE 550/10) (c), the mass has slightly high signal intensity compared to the myometrium. Tiny scattered high signal intensity areas (arrowheads), presenting hemorrhagic foci are seen. Gadolinium-enhanced fat suppressed turbo spin-echo T1-weighted axial image (d) shows diffuse and strong enhancement of the solid portion of the mass.
The mass shows high signal intensity on axial high b-value image (b=1000 s/mm2) (e), but ADC map (f) revealed no diffusion restriction (asterisk). Photograph of gross surgical specimen (g) demonstrates a large yellowish polypoid mass. Small cystic areas scattered within the mass can be observed on the cut surface.
References
1. Clement PB, Scully RE. Mullerian adenosarcoma of the uterus: a clinicopathologic analysis of 100 cases with a review of the literature. Hum Pathol. 1990; 21:363–381.
2. Chourmouzi D, Boulogianni G, Zarampoukas T, Drevelengas A. Sonography and MRI of tamoxifen-associated müllerian adenosarcoma of the uterus. AJR Am J Roentgenol. 2003; 181:1673–1675.
3. Yoshizako T, Wada A, Kitagaki H, Ishikawa N, Miyazaki K. MR imaging of uterine adenosarcoma: case report and literature review. Magn Reson Med Sci. 2011; 10:251–254.
4. Lee HK, Kim SH, Cho JY, Yeon KM. Uterine adenofibroma and adenosarcoma: CT and MR findings. J Comput Assist Tomogr. 1998; 22:314–316.
5. Soh E, Eleti A, Jimenez-Linan M, Arends M, Latimer J, Sala E. Magnetic resonance imaging findings of tamoxifen-associated uterine Mullerian adenosarcoma: a case report. Acta Radiol. 2008; 49:848–851.
6. Takeuchi M, Matsuzaki K, Yoshida S, et al. Adenosarcoma of the uterus: magnetic resonance imaging characteristics. Clin Imaging. 2009; 33:244–247.
7. Chi F, Wu R, Zeng Y, Xing R, Liu Y, Xu Z. Effects of toremifene versus tamoxifen on breast cancer patients: a meta-analysis. Breast Cancer. 2013; 20:111–122.
8. Clement PB. Müllerian adenosarcomas of the uterus with sarcomatous overgrowth. A clinicopathological analysis of 10 cases. Am J Surg Pathol. 1989; 13:28–38.
9. Nalaboff KM, Pellerito JS, Ben-Levi E. Imaging the endometrium: disease and normal variants. Radiographics. 2001; 21:1409–1424.
10. Shah SH, Jagannathan JP, Krajewski K, O'Regan KN, George S, Ramaiya NH. Uterine sarcomas: then and now. AJR Am J Roentgenol. 2012; 199:213–223.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download