Abstract
Purpose
This report compared the diagnostic effectiveness between ultrasmall superparamagnetic iron oxide (USPIO) and gadolinium (Gd) based magnetic resonance imaging (MRI) for differentiation of axillary status in breast cancer patients.
Materials and Methods
The present authors performed a meta-analysis of previous studies that compared USPIO or Gd based MRI with histological diagnosis after surgery or biopsy. We searched PubMed, EMBASE, Cochrane Library, ScienceDirect, SpringerLink, Ovid databases and references of articles to identify studies reporting data until December 2013. Pooled sensitivity and specificity were calculated for every study; summary receiver operating characteristic and subgroup analysis was done. Analyses of study quality and heterogeneity were also assessed.
Results
There were 14 publications that met the criteria for inclusion in our metaanalysis. USPIO based MRI showed 0.83 (95% CI: 0.75-0.89) and 0.97 (95% CI: 0.94-0.98) for pooled sensitivity and specificity, respectively. Gd based MRI represented pooled sensitivity and specificity of 0.61 (95% CI: 0.55-0.67) and 0.90 (95% CI: 0.87-0.92) for each. Overall weighted area under the curve for USPIO and Gd based MRI were 0.9563 and 0.9051, respectively.
Conclusion
USPIO based MRI had a tendency toward high pooled sensitivity and specificity in detection of axillary metastases for breast cancer. This result may mean that USPIO based MRI could be used as complementary modality to differentiate axillary status more precisely, and assist in the decision-making process regarding possible invasive procedures, such as sentinel node biopsy.
Breast cancer is the third leading cause of death after lung and colorectal cancer in United States (1). It preferentially tends to metastasize to axillary lymph nodes (2). Traditionally, axillary lymph node dissection was performed to decide the stage of the disease and the planning of postsurgical treatment (3, 4). However, this method can cause many complications, such as lymphedema, nerve injury, shoulder movement disorder and so on (3, 4). Recently, sentinel lymph node biopsy has been performed for the assessment of axillary lymph node status (3, 4). Although sentinel lymph node biopsy reduced the incidence of complications by minimizing operative area, it still has a risk of surgical complications (5). Therefore, many attempts have been made to decide the non-surgical preoperative staging of axillary lymph nodes with multiple imaging modalities (6, 7).
Magnetic resonance imaging (MRI) is one of representative imaging modalities for assessment of metastasis in cancer patients (8). It is a non-ionizing imaging technique which generate the images by using hydrogen nuclei reaction within the body in the magnetic field (8). MRI is also effective in the identification of the axillary lesions, and able to give additional information by using intravascular contrast media such as gadolinium (Gd) chelate (8). In recent years, several research about detection of lymph node metastasis using nanoparticles have been actively performed, especially ultrasmall superparamagnetic iron oxide (USPIO) based MRI (9, 10, 11, 12). USPIO is inorganic nanoparticle that the core is composed of metal molecules of iron oxide (13). There are two distinct pathways that could explain about distribution in the lymph node (14). The first is direct transcapillary passage after intravenous injection that passes through the medullary sinus of lymph node followed by phagocytosis. The second is transportation to the afferent lymphatic channel through draining lymphatic vessel after interstitial injection (14). In normal lymph nodes, as USPIO is taken up by macrophages, it acts as negative imaging contrast agent when using T2 and T2* MRI which produces dark signals (13). However, in cases of the metastatic lymph nodes, USPIO based MRI shows white regions as macrophages are replaced by tumor cells in these nodes (13). In hence, USPIO based MRI could potentially identify lymphatic metastases of malignant tumors.
In this study, we used a meta-analysis to assess the diagnostic effectiveness between USPIO and Gd based MRI as a predictor of axillary metastasis in breast cancer using axillary histology as the reference standard.
To compare the diagnostic effectiveness between USPIO and Gd based MRI for the detection of axillary metastasis in breast cancer patients, we searched PubMed, EMBASE, Cochrane Library, ScienceDirect, SpringerLink, Ovid databases up to December 2013. The searched keywords were as follows; "magnetic resonance imaging AND axilla", "ultrasmall superparamagnetic iron oxide AND axilla" and "gadolinium AND axilla". References of articles were also searched manually. Searches were not restricted according to publication area.
Every investigator planned and reviewed the study design. The investigators independently reviewed every searched article, and determined whether the articles were suitable for the following inclusion criteria: 1) original article, 2) publication in English, 3) histologic confirmation as reference standard for diagnosis of axillary lymph node metastasis obtained with core needle biopsy, sentinel node biopsy or axillary lymph node dissection, 4) numbers of true positive (TP), false positive (FP), false negative (FN) and false positive (FP) were reported or could be calculated with "patient to patient" or "node to node" data. Every review article, editorial letter, case report, animal testing research paper, poster presentation, and non-English article were discarded. In addition, when the study had no comparison with breast histology, and had no description whether to use contrast media or not, it was excluded in the current analysis of this study. The investigators were unblinded to information about the author names, research affiliations and the journal titles. If there was disagreement about the selection of studies, we discussed until consensus was achieved.
The authors verified the qualities of the included studies by using the quality assessment of diagnostic accuracy studies (QUADAS) tool which has 14 checklists. Each checklist was counted as one point when it could be checked as "yes".
The endpoint of this study was to compare the diagnostic effectiveness using sensitivity and specificity between USPIO and Gd based MRI. Sensitivity and specificity were either described in the studies or were calculable from the analysis of the numbers of TP, TN, FN and FP.
TP was defined as the number of patients that had axillary metastasis on MRI and corresponding metastatic lymph nodes on subsequent histologic findings. The number of FP was patients who had positive nodes on MRI with negative findings on histological analysis. The frequency of FN was patients who had negative findings on MRI but metastatic nodes on histologic examination. TN was calculated as the number of patients with negative nodes on histology among the patients with negative findings on MRI.
In this research, we used Cochran's Q and I2 statistics to analyze the heterogeneity of the included studies. When the p-value was less than 0.1, it suggested that heterogeneity existed between studies (15). In addition, heterogeneity was differentiated into 3 categories depending on the range of I2 index (i.e., < 25%: low, 25-75%: moderate, > 75%: high) (16). If heterogeneity existed, random effect model was used to account for variation within studies.
We used summary receiver operating characteristics (SROC) curves to present the results quantitatively. In addition, the present authors used diagnostic odds ratio (DOR) and area under curve (AUC) to compare diagnostic values for each type of contrast media. Subgroup analysis was performed to explore the sources of heterogeneity between studies as following parameters: publication area, publication year, magnetic field strength, number of patient, coil type, QUADAS score and index test.
Every statistical analysis was done by using Meta-Disc 1.4 (Clinical BioStatistics Unit, Hospital Universitario Ramón y Cajal, Madrid, Spain) and STATA 12.0 (STATA Co., College Station, Texas, USA). A P < 0.05 was considered as statistically significant.
Figure 1 shows the pertinent selection process of this meta-analysis. A total of 931 articles were found by using the keywords that described above. Finally, 14 articles were selected and analyzed. The duplicated articles were judged based on the authors, titles and contents.
Table 1 shows characteristics and quality of included studies. Every study included in this meta-analysis was published between 1997 and 2013. All of the included studies were more than 7 QUADAS points, and 8 studies were more than 10 QUADAS points. Based on contrast media, all studies included in this meta-analysis were divided into USPIO and Gd based MRI. In USPIO based MRI, every study was USPIO enhanced MRI which compared signal intensity between pre- and post-contrast injection (17, 18, 19, 20, 21, 22, 23). If there was an increase in signal intensity, the axillary node was defined as metastasis. With regard to Gd based MRI, the included research dichotomized into Gd enhanced MRI and Dynamic Gd enhanced MRI. In Gd enhanced MRI, axillary metastasis was determined through the comparison of change in the signal intensity similar to USPIO enhanced MRI (24, 25, 26, 27). Unlike that, when the enhanced pattern of axillary lymph node was "plateau type" or "washout type", it was determined as metastatic lymph node in dynamic Gd enhanced MRI (28, 29, 30).
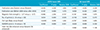
The pooled sensitivity and specificity for MRI were 0.68 (95% CI: 0.63 - 0.72) and 0.92 (95% CI: 0.90-0.93). We dichotomized MRI into USPIO and Gd based MRI according to contrast media. USPIO based MRI showed 0.83 (95% CI: 0.75 - 0.89) and 0.97 (95% CI: 0.94-0.98) for pooled sensitivity and specificity, respectively. In Gd based MRI, pooled sensitivity and specificity were 0.61 (95% CI: 0.55-0.67) and 0.90 (95% CI: 0.87-0.92) for each (Fig. 2).
Figure 3 shows SROC curves and Q indices of MRI, USPIO and Gd based MRI. The areas under curves (AUCs) of each study were calculated to compare the diagnostic precision of each modality. AUC and Q index for MRI were 0.9286 and 0.8635, respectively. USPIO based MRI showed AUC of 0.9563, and Q index of 0.8991. In Gd based MRI, AUC and Q index were 0.9051 and 0.8367 for each.
Table 2 shows study heterogeneity according to contrast media. With regard to USPIO based MRI, both the sensitivity (P = 0.0062, I2 index = 66.7%) and specificity (P = 0.0987, I2 index = 43.8%) were moderately heterogeneous. In Gd based MRI, sensitivity and specificity showed high heterogeneity (P = 0.0000, I2 index = 91.0% and P = 0.0000, I2 index = 88.1% for each).
Tables 3 and 4 show the subgroup analysis for USPIO and Gd based MRI. Subgroup analysis of USPIO based MRI showed that the publication year (before 2009) achieved the highest diagnostic accuracy for detection of axillary metastasis (0.91 (95% CI: 0.81-0.96) and 0.93 (95% CI: 0.84-0.98) for pooled sensitivity and specificity, respectively). In Gd based MRI, subgroup analysis also represented that the publication year (before 2009) obtained the highest diagnostic precision for differentiation of axillary status (pooled sensitivity and specificity of 0.89 [95% CI: 0.80-0.95] and 0.76 [95% CI: 0.67-0.84] for each). The authors also attempted to analyze the diagnostic effectiveness according to cancer stage and patient age, but it was not implemented due to lack of data.
In USPIO and Gd based MRI, meta-regression analysis was performed to find out the sources of heterogeneity that include publication year (before 2009 versus after 2010), publication area (Eastern versus Western), magnetic field strength (< 1.5T versus ≥ 1.5T), number of patient (< 30 versus ≥ 30), coil type (body versus breast), QUADAS score (< 10 versus ≥ 10) and index test (Gd enhanced versus dynamic Gd enhanced; applied to Gd based MRI only) (Table 5). With respect to USPIO based MRI, publication year (P = 0.000, relative DOR = 1.375), number of patients (P = 0.000, relative DOR = 0.529), coil type (P = 0.000, relative DOR = 1.202) and QUADAS score (P = 0.000, relative DOR = 0.351) were related to heterogeneity. In Gd based MRI, publication area (P = 0.000, relative DOR = 0.239), magnetic field strength (P = 0.006, relative DOR = 1.974), QUADAS score (P = 0.000, relative DOR = 4.330), coil type (P = 0.000, relative DOR = 1.605) and index test (P = 0.000, relative DOR = 1.138) were associated with heterogeneity.
In this study, we compared and analyzed diagnostic effectiveness of MRI in the judgment of axillary lymph node metastases in breast cancer patients through the analysis of pooled sensitivity and specificity. We identified studies that compared USPIO or Gd based MRI with histological diagnosis after surgery or biopsy. Finally, 14 studies that included 979 patients were subjected to this analysis.
This meta-analysis included clinical research that published up to December 2013, so any other studies published after that date were not subsumed. Within the included period, the authors strived to minimize the loss of data. Although our institution is not authorized in every medical database, we searched the authorized databases as many as possible, such as PubMed, EMBASE, Cochrane Library, ScienceDirect, SpringerLink and Ovid databases. In addition, the authors checked every reference list manually.
This meta-analysis revealed that MRI had high pooled specificity of 0.91 (95% CI: 0.78-0.93) and low pooled sensitivity of 0.68 (95% CI: 0.63-0.73) for axillary metastasis. However, USPIO based MRI showed higher diagnostic accuracy than Gd based MRI (pooled sensitivity of 0.83 [95% CI: 0.75-0.89] versus 0.62 [95% CI: 0.55-0.68]; pooled specificity of 0.97 [95% CI: 0.94-0.98] versus 0.89 [95% CI: 0.86-0.91]). This result may mean that USPIO based MRI is superior to Gd based MRI in the assessment of axillary lymph node metastases in patients with breast cancers.
In this study, we used QUADAS score to evaluate the quality of included studies. Although every study included in this meta-analysis showed more than 7 QUADAS score, heterogeneity was found among the included studies. In USPIO and Gd based MRI, both of sensitivity and specificity of the included studies were heterogeneous. So, we performed meta-regression analysis to evaluate the sources of heterogeneity. The authors found that publication year, number of patients, coil type and QUADAS score were significant sources of heterogeneity for USPIO based MRI. In addition, publication area, magnetic field strength, coil type, QUADAS score and index test were identified as significant sources of heterogeneity for Gd based MRI.
Furthermore, we performed subgroup analysis to find out the effect of characteristics and quality of the studies on the diagnostic accuracy of axillary metastasis in USPIO and Gd based MRI. For both of USPIO and Gd based MRI, publication year (before 2009) significantly affect the diagnostic value.
As mentioned above, USPIO based MRI showed higher diagnostic accuracy than Gd based MRI in this meta-analysis. Although both of USPIO and Gd based MRI showed heterogeneity for sensitivity and specificity, we could identify that USPIO based MRI had higher diagnostic value than Gd based MRI by using AUC with SROC analysis.
The meta-analysis we have described in this article has its own limitation. Although most studies showed both of "patient to patient" data and "node to node (lesion to lesion)" data, some research presented "patient to patient" data or "node to node (lesion to lesion)" data only. In this study, we preferred to analyze the "patient to patient" data rather than "node to node (lesion to lesion)" data. In some studies, however, as there were no "patient to patient" data, "node to node (lesion to lesion)" data were applied to the analysis of diagnostic accuracy. So, we think that the results of this study may have been affected. In the studies which showed both of "patient to patient" data and "node to node (lesion to lesion)" data, however, the sensitivity and specificity showed no large numerical differences between each data. Thus, although we think that this limitation may hardly result in large bias, additional studies may be required.
In conclusion, USPIO based MRI is more effective diagnostic imaging modality for axillary metastases with higher pooled sensitivity and specificity than Gd based MRI. Therefore, USPIO based MRI could be used as complementary modality to differentiate axillary status more precisely, and assist in the decision-making process regarding possible invasive procedures, such as sentinel node biopsy.
Figures and Tables
Fig. 1
Flow chart of this meta-analysis.
CI = confidence interval; Gd = gadolinium; MRI = magnetic resonance imaging; USPIO = ultrasmall superparamagnetic iron oxide
Fig. 2
Forest plots of all studies.
Sensitivity and specificity of (a) overall MRI, (b) USPIO and (c) Gd based MRI. CI = confidence interval; Gd = gadolinium; MRI = magnetic resonance imaging; USPIO = ultrasmall superparamagnetic iron oxide

Fig. 3
Summary receiver operating characteristics curves of (a) overall MRI, (b) USPIO and (c) Gd based MRI. AUC = area under curve; Gd = gadolinium; MRI = magnetic resonance imaging; SE = standard error; SROC = summary receiver operating characteristic; USPIO = ultrasmall superparamagnetic iron oxide

Table 1
Characteristics and Quality of the Included Studies

Table 2
Test for Heterogeneity According to Contrast Media

| Contrast media | χ2 | P value | I2 index (%) |
|---|---|---|---|
| USPIO | |||
| Sensitivity | 18.02 | 0.0062 | 66.7 |
| Specificity | 10.68 | 0.0987 | 43.8 |
| Gadolinium | |||
| Sensitivity | 66.79 | 0.0000 | 91.0 |
| Specificity | 50.62 | 0.0000 | 88.1 |
Table 3
Summary Estimates of Pooled Sensitivity and Specificity for USPIO Based MRI Studies

Table 4
Summary Estimates of Pooled Sensitivity and Specificity for Gd Based MRI Studies

Table 5
Meta-Regression for the Potential Source of Heterogeneity
References
1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014; 64:9–29.
2. Wiechmann L, Sampson M, Stempel M, et al. Presenting features of breast cancer differ by molecular subtype. Ann Surg Oncol. 2009; 16:2705–2710.
3. Wilking N, Rutqvist LE, Carstensen J, Mattsson A, Skoog L. Stockholm Breast Cancer Study Group. Prognostic significance of axillary nodal status in primary breast cancer in relation to the number of resected nodes. Acta Oncol. 1992; 31:29–35.
4. Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst. 2006; 98:599–609.
5. Purushotham AD, Upponi S, Klevesath MB, et al. Morbidity after sentinel lymph node biopsy in primary breast cancer: results from a randomized controlled trial. J Clin Oncol. 2005; 23:4312–4321.
6. Robertson IJ, Hand F, Kell MR. FDG-PET/CT in the staging of local/regional metastases in breast cancer. Breast. 2011; 20:491–494.
7. Taylor K, O'Keeffe S, Britton PD, et al. Ultrasound elastography as an adjuvant to conventional ultrasound in the preoperative assessment of axillary lymph nodes in suspected breast cancer: a pilot study. Clin Radiol. 2011; 66:1064–1071.
8. Cooper KL, Meng Y, Harnan S, et al. Positron emission tomography (PET) and magnetic resonance imaging (MRI) for the assessment of axillary lymph node metastases in early breast cancer: systematic review and economic evaluation. Health Technol Assess. 2011; 15:iii–iv. 1–134.
9. Deserno WM, Harisinghani MG, Taupitz M, et al. Urinary bladder cancer: preoperative nodal staging with ferumoxtran-10-enhanced MR imaging. Radiology. 2004; 233:449–456.
10. Keller TM, Michel SC, Frohlich J, et al. USPIO-enhanced MRI for preoperative staging of gynecological pelvic tumors: preliminary results. Eur Radiol. 2004; 14:937–944.
11. Koh DM, Brown G, Temple L, et al. Rectal cancer: mesorectal lymph nodes at MR imaging with USPIO versus histopathologic findings--initial observations. Radiology. 2004; 231:91–99.
12. Harisinghani MG, Barentsz J, Hahn PF, et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med. 2003; 348:2491–2499.
13. Hudgins PA, Anzai Y, Morris MR, Lucas MA. Ferumoxtran-10, a superparamagnetic iron oxide as a magnetic resonance enhancement agent for imaging lymph nodes: a phase 2 dose study. AJNR Am J Neuroradiol. 2002; 23:649–656.
14. Weissleder R, Elizondo G, Wittenberg J, Rabito CA, Bengele HH, Josephson L. Ultrasmall superparamagnetic iron oxide: characterization of a new class of contrast agents for MR imaging. Radiology. 1990; 175:489–493.
15. Fleiss JL. Analysis of data from multiclinic trials. Control Clin Trials. 1986; 7:267–275.
16. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–560.
17. Memarsadeghi M, Riedl CC, Kaneider A, et al. Axillary lymph node metastases in patients with breast carcinomas: assessment with nonenhanced versus uspio-enhanced MR imaging. Radiology. 2006; 241:367–377.
18. Nakai G, Matsuki M, Harada T, et al. Evaluation of axillary lymph nodes by diffusion-weighted MRI using ultrasmall superparamagnetic iron oxide in patients with breast cancer: initial clinical experience. J Magn Reson Imaging. 2011; 34:557–562.
19. Kimura K, Tanigawa N, Matsuki M, et al. High-resolution MR lymphography using ultrasmall superparamagnetic iron oxide (USPIO) in the evaluation of axillary lymph nodes in patients with early stage breast cancer: preliminary results. Breast Cancer. 2010; 17:241–246.
20. Harada T, Tanigawa N, Matsuki M, Nohara T, Narabayashi I. Evaluation of lymph node metastases of breast cancer using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging. Eur J Radiol. 2007; 63:401–407.
21. Michel SC, Keller TM, Frohlich JM, et al. Preoperative breast cancer staging: MR imaging of the axilla with ultrasmall superparamagnetic iron oxide enhancement. Radiology. 2002; 225:527–536.
22. Stadnik TW, Everaert H, Makkat S, Sacre R, Lamote J, Bourgain C. Breast imaging. Preoperative breast cancer staging: comparison of USPIO-enhanced MR imaging and 18F-fluorodeoxyglucose (FDC) positron emission tomography (PET) imaging for axillary lymph node staging--initial findings. Eur Radiol. 2006; 16:2153–2216.
23. Stets C, Brandt S, Wallis F, Buchmann J, Gilbert FJ, Heywang-Kobrunner SH. Axillary lymph node metastases: a statistical analysis of various parameters in MRI with USPIO. J Magn Reson Imaging. 2002; 16:60–68.
24. Schipper RJ, Smidt ML, van Roozendaal LM, et al. Noninvasive nodal staging in patients with breast cancer using gadofosveset-enhanced magnetic resonance imaging: a feasibility study. Invest Radiol. 2013; 48:134–139.
25. Mumtaz H, Hall-Craggs MA, Davidson T, et al. Staging of symptomatic primary breast cancer with MR imaging. AJR Am J Roentgenol. 1997; 169:417–424.
26. Baltzer PA, Dietzel M, Burmeister HP, et al. Application of MR mammography beyond local staging: is there a potential to accurately assess axillary lymph nodes? Evaluation of an extended protocol in an initial prospective study. AJR Am J Roentgenol. 2011; 196:W641–W647.
27. Hwang SO, Lee SW, Kim HJ, Kim WW, Park HY, Jung JH. The comparative study of ultrasonography, contrast-enhanced MRI, and (18)F-FDG PET/CT for detecting axillary lymph node metastasis in T1 breast cancer. J Breast Cancer. 2013; 16:315–321.
28. Kvistad KA, Rydland J, Smethurst HB, Lundgren S, Fjosne HE, Haraldseth O. Axillary lymph node metastases in breast cancer: preoperative detection with dynamic contrast-enhanced MRI. Eur Radiol. 2000; 10:1464–1471.
29. Murray AD, Staff RT, Redpath TW, et al. Dynamic contrast enhanced MRI of the axilla in women with breast cancer: comparison with pathology of excised nodes. Br J Radiol. 2002; 75:220–228.
30. Valente SA, Levine GM, Silverstein MJ, et al. Accuracy of predicting axillary lymph node positivity by physical examination, mammography, ultrasonography, and magnetic resonance imaging. Ann Surg Oncol. 2012; 19:1825–1830.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download