Abstract
Arteriovenous malformation (AVM) of the pancreas is extremely rare, although it may be increasingly diagnosed due to the widespread use of cross-sectional imaging of the abdomen. Early diagnosis of this disease is important to prevent delay of treatment and resulting fatal complications. We report a rare case of pancreatic AVM in a 48-year-old man who presented with severe chronic anemia and early gastric cancer, which made diagnosis challenging. Imaging findings, including ultrasound, computed tomography, and magnetic resonance imaging, are shown, as well as the pathologic features.
Anemia is one of the most common causes of dizziness. Most chronic anemia is associated with gastrointestinal bleeding, such as peptic ulcers or cancer of various organs (1). Pancreatic arteriovenous malformation (AVM) is a rare cause of gastrointestinal bleeding (2). They are usually asymptomatic but may present with abdominal pain, portal hypertension in the absence of hepatopathy, or gastrointestinal bleeding. Although pancreatic AVM is rare, it should be considered in patients with anemia of unknown origin or those who failed therapy for more common causes of anemia.
Even though previous studies have reported the imaging findings of pancreatic AVM including ultrasound, computed tomography, and magnetic resonance imaging, most reports include only a single or limited number of imaging modalities.
We report a case of pancreatic AVM in a patient with early gastric cancer (EGC) and severe chronic anemia, which was successfully managed with pylorus-preserving pancreaticoduodenectomy (PPPD) which made pathologic correlation possible. Concomitant EGC made the diagnosis of AVM challenging. Multimodality radiologic findings, emphasizing magnetic resonance imaging (MRI), and the pathologic correlation are presented.
A 47-year-old man was referred to our hospital to determine the cause of his dizziness. He was diagnosed with an iron deficiency anemia of unknown origin 2 years prior to admission at our hospital. His hemoglobin level decreased to 6.2 g/dL. Coagulation profile was within the normal range, with the international normalized ratio (INR) of 1.01, activated partial thromboplastin time of 24.4 s, and platelets of 367 × 103/µL. Other laboratory data were grossly normal, apart from elevated levels of total amylase of 279 U/L, lipase of 724 IU/L, and total bilirubin of 2.00 mg/dL. The patient tested negative for hepatitis B and C viruses. Upper gastrointestinal (UGI) endoscopy showed an approximately 0.5 cm flat, depressed lesion in the lesser curvature of the antrum of the stomach that was confirmed as an adenocarcinoma using an endoscopic biopsy.
He underwent contrast-enhanced multidetector computed tomography (Sensation 64; Siemens Healthcare, Erlangen, Germany) of the abdomen at that time. The CT scan obtained during the arterial phase revealed an irregular shaped hypervascular lesion of 6.1 × 2.2 cm in the pancreatic head without evidence of vascular or adjacent organ invasion. In the portal venous phase, this lesion was slightly more enhanced relative to the normal pancreatic parenchyma. It was difficult to define because of isodense enhancement during the delayed phase (Fig. 1).
For further evaluation, he underwent MRI of the abdomen using a 3.0-Tesla (T) MRI system (Achieva; Philips Medical System, Bothell, WA, USA) using the parameters presented in Table 1. On T2-weighted images, clustered tubular structures and dilated pancreaticoduodenal vein demonstrating characteristic signal void, indicating the presence of rapid blood flow. This lesion did not show mass effect or dilation of the main pancreatic duct. After an intravenous injection of a paramagnetic contrast agent, the enhancement observed was similar to that of the aorta. Dynamic T1-weighted imaging during the arterial phase showed early filling of the proximal portions of the superior mesenteric vein and superior and inferior pancreaticoduodenal veins that drained into the dilated portal vein (Fig. 2).
For further hemodynamic evaluation, a noninvasive Doppler ultrasonography (iU22 unit; Philips Medical System, Bothell, WA, USA) was performed. Color Doppler ultrasonography showed a mosaic pancreatic color flow pattern with dilatation of the hepatic portal vein and pancreaticoduodenal vein. On spectral Doppler ultrasonography, we found arterial waveforms with increased velocity showing pulsatile pattern, indicating the draining role of the portal vein (Fig. 3).
These clinical and radiologic findings led to the diagnosis of EGC and pancreatic AVM. The clinician thought that his chronic anemia had arisen from EGC because cancer is one of the most common causes of chronic gastrointestinal bleeding. He subsequently underwent endoscopic submucosal dissection (ESD). After treatment of EGC, we expected that his symptoms, and laboratory profile related to anemia, would improve. However, laboratory findings (including hemoglobin levels) did not normalize. Immediately after ESD, the patient's hemoglobin level was 8.6 g/dL, but 9 months after ESD, it had reduced to 4.5 g/dL.
On follow-up UGI endoscopy, intermittent bleeding was suspected based on a petechial lesion and pale mucosa in the second portion of the duodenum without an active bleeding focus. We concluded that the cause of duodenal bleeding was a pancreatic AVM located in the head of the pancreas.
At the same time, there were no clinical signs of portal hypertension which was a good indication of early surgical removal and a good prognosis. Although transcatheter arterial embolization with n-butyl-2-cyanoacrylate could be another treatment option, surgical resection of the affected organ was recommended as the only curative treatment in a patient with good indications (3). After sufficient discussion between the patient, clinicians, and radiologists, the patient underwent PPPD, and the surgical findings included a bulky pancreatic head with multiple collaterals. Despite massive intraoperative bleeding, the pancreatic mass was successfully removed. Multiple honeycomb-like dilated spaces were found in the pancreatic head and the uncinate process in the surgical specimen (Fig. 4). Histopathologic examination of the resected pancreas showed irregularly dilated angiodysplastic vessels, composed of a thick-walled artery and thin-walled vein connections with irregular duplication (Fig. 4). Finally, the sample was pathologically confirmed as a pancreatic AVM.
The patient recovered uneventfully. Immediately after PPPD, the patient's hemoglobin level reached 8.1 g/dL and gradually improved during follow-up. The patient has been well for 1 year since the operation, and his hemoglobin level normalized at 13.2 g/dL without complications.
Pancreatic AVM is very rare. According to Meyer et al. (4), the most common site of AVM is the cecum and right colon (78%), followed by the jejunum (10.5%), whereas only 0.9% of all AVMs are found in the pancreas (4). The cause of pancreatic AVM is thought to be congenital in 90% of cases, and 10% to 30% of pancreatic AVM are associated with Osler-Weber-Rendu disease (3). Several cases of acquired pancreatic AVM occurred because of pancreatitis, trauma, and tumors. As our patient had no history of pancreatitis or liver cirrhosis, the pancreatic AVM was thought to be congenital.
The most common presentation of pancreatic AVM is gastrointestinal bleeding. Patients with pancreatic AVM can also present with abdominal pain, caused by the shunting of blood away from the mesenteric circulation through the AVM. Jaundice, secondary to hemobilia, is a possible, but rare, clinical manifestation of AVM. According to a prior report (5), gastrointestinal bleeding can be caused by duodenal ulcer or duodenitis bleeding associated with the pancreatic AVM, direct bleeding from the pancreatic AVM to the pancreatic duct or bile duct, or bleeding from esophageal or gastric varices associated with portal hypertension. Among these causes, a duodenal ulcer or duodenitis appeared to have risen from regional ischemia caused by the diseased mucosa (6).
In our case, the patient complained only of dizziness without any abdominal pain or jaundice, and there was no direct evidence of gastrointestinal bleeding, such as melena or hematochezia, preoperatively. However, chronic bleeding might have occurred, considering the low hemoglobin level. Concomitant EGC contributed to the delayed diagnosis of the exact cause of the chronic anemia, even though it was a small lesion, because cancer is one of the most common causes of anemia. Although there was no direct evidence of massive gastrointestinal bleeding, chronic occult blood loss might have occurred from a petechial lesion in the second portion of the duodenum associated with pancreatic AVM, considering improvement of his laboratory profile after PPPD.
Pancreatic AVM is diagnosed using CT, MRI, color Doppler ultrasonography, and angiography. Contrast-enhanced dynamic CT shows multiple, pronounced enhancements of small hypervascular spots in the lesion. Based on these CT findings, hypervascular pancreatic cancer cannot be ruled out in the differential diagnosis, especially for inexperienced radiologists. In such a case, characteristic signal void on T1- and T2-weighted imaging could provide the diagnostic clue for pancreatic AVM. This signal void is a characteristic of rapid blood flow (7). After enhancement, early contrast filling of the enlarged portal venous system was seen in the arterial phase. In addition, demonstration of enhancement of the lesion, commensurate with the aorta, on contrast enhanced multiphasic imaging is helpful in diagnosing pancreatic AVM (2). On the other hand, color Doppler ultrasonography shows a delineated hypervascular, hypoechoic mass with a mosaic color flow pattern at the site of the malformation that is helpful in evaluating hemodynamic changes. One of the most important findings on spectral Doppler ultrasound is a "pulsatile wave form with high flow velocity" of a draining portal vein system (8).
Angiography plays an important role in confirmatory examination, as well as planning treatment (7). The angiographic findings include dilated and tortuous feeding arteries, a racemose intratumoral vascular network, followed by a transient avid pancreatic stain, early venous filling into the portal vein, and early wash-out of the pancreatic stain (9). However, these findings are also observed in other conditions such as pancreatitis or hypervascular neoplasms (10). We did not perform angiography on our patient because we planned surgical resection, and the patient did not want to undergo an invasive angiographic study.
Management of pancreatic AVM may involve surgical and conservative therapy, such as arterial embolization, irradiation, or portovenous shunts. Nishiyama et al. (3) recommended total surgical resection of the affected organ as the only curative treatment. If the lesion is surgically removed at an early stage, prior to the patient developing portal hypertension, the patient has a good prognosis. Although surgical resection is the treatment of choice, it has a limited role in treating patients with a large, complicated pancreatic AVM, as they have a high risk of massive intraoperative bleeding (8). Fortunately, our patient was an appropriate surgical candidate, and subsequently underwent surgical resection. He recovered uneventfully and has not experienced gastrointestinal bleeding since the surgery.
Although several previous reports offer established imaging findings of pancreatic AVM, unlike our case, these reports provide single or limited imaging modalities. Because our patient underwent MRI, CT, and noninvasive Doppler ultrasonography for evaluation, we were able to correlate several findings of the lesion between multimodalities and made a comprehensive conclusion before surgical treatment. It is very important for inexperienced radiologists to remember that pancreatic AVM is easily differentiated from hypervascular mass on CT images when using signal void on a T2-weighted MR image. Furthermore, we can correlate these various findings of multimodalities directly with pathology after PPPD. Because most patients with pancreatic AVM are treated with arterial embolization using n-butyl-2-cyanoacrylate, rather than PPPD, our report is meaningful and presents a rare case of a multimodality imaging spectrum with pathologic correlation.
In summary, although pancreatic AVM is a rare disease, it should be included in the differential diagnosis as a cause of chronic anemia of unknown origin, or for therapeutic failure for more common causes of anemia. Familiarity with imaging findings of pancreatic AVM on CT, MRI, and ultrasonography, as shown in this case, will aid in the early diagnosis in uncertain cases. In addition, signal void on MRI is a diagnostic clue in cases of pancreatic hypervascular lesions.
Figures and Tables
Fig. 1
Pancreatic arteriovenous malformation in a 41-year-old man, CT findings. (a) Axial scan, during the arterial phase, shows an irregularly tangled hypervascular lesion in the pancreatic head. (b) In the delayed phase, the lesion is difficult to define because of isodense enhancement relative to normal pancreatic parenchyma.
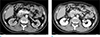
Fig. 2
Pancreatic arteriovenous malformation in a 41-year-old man, MRI findings. (a, b) T2-weighted imaging shows the characteristic clustered tubular signal void (arrows). (c-e) Axial scan during the arterial phase of dynamic T1-weighted imaging shows an irregularly tangled hypervascular lesion in the pancreatic head (c) and early enhancement of the dilated portal vein (d) and pancreaticoduodenal vein (arrow) that drained into the dilated portal vein (e).
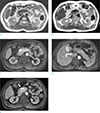
Fig. 3
Pancreatic arteriovenous malformation in a 41-year-old man, US findings. (a) A gray-scale ultrasonogram demonstrates an ill-marginated anechoic lesion (arrows) around the head of the pancreas. (b) On color Doppler ultrasonogram shows mosaic color flow pattern with large amount of color signals around the pancreas. (c) Spectral Doppler ultrasonogram of the main portal vein reveals pulsatile pattern, suggesting the draining role of the portal vein.
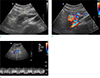
Fig. 4
Pancreatic arteriovenous malformation in a 41-year-old man, gross and microscopic findings. (a) A cut section of the pancreas shows multiple honeycomb-like dilated spaces. (b, c) Histopathologic examination of the resected pancreas reveals irregularly dilated angiodysplastic vessels in the pancreas (× 40, H&E staining) (b) and thick-walled artery and thin-walled vein connections with irregular duplication (arrows) (× 100, elastin staining) (c).
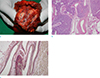
Table 1
MR Imaging Sequence and Parameters

2D = two dimensional; CE-T1WI = contrast enhanced-T1 weighted imaging; DWI = diffusion weighted imaging; FA = flip angle; FOV = field of view; MRCP = magnetic resonance cholangiopancreatography; T2WI = T2 weighted imaging; TE = echo time; TR = repetition time
*CE-T1WI was acquired after the administration of Gadoxetate disodium (Primovist®, Gd-EOB-DTPA, Bayer Schering Pharma, Berlin, Germany) at a dose of 0.1 mmol/kg and an injection rate of 1 mL/sec.
References
1. Rockey DC. Occult gastrointestinal bleeding. N Engl J Med. 1999; 341:38–46.
2. Hansen W, Maximin S, Shriki JE, Bhargava P. Multimodality imaging of pancreatic arteriovenous malformation. Curr Probl Diagn Radiol. 2015; 44:105–109.
3. Nishiyama R, Kawanishi Y, Mitsuhashi H, et al. Management of pancreatic arteriovenous malformation. J Hepatobiliary Pancreat Surg. 2000; 7:438–442.
4. Meyer CT, Troncale FJ, Galloway S, Sheahan DG. Arteriovenous malformations of the bowel: an analysis of 22 cases and a review of the literature. Medicine (Baltimore). 1981; 60:36–48.
5. Aida K, Nakamura H, Kihara Y, Abe S, Okamoto K, Otsuki M. Duodenal ulcer and pancreatitis associated with pancreatic arteriovenous malformation. Eur J Gastroenterol Hepatol. 2002; 14:551–554.
6. Koito K, Namieno T, Nagakawa T, et al. Congenital arteriovenous malformation of the pancreas: its diagnostic features on images. Pancreas. 2001; 22:267–273.
7. Makhoul F, Kaur P, Johnston TD, Jeon H, Gedaly R, Ranjan D. Arteriovenous malformation of the pancreas: a case report and review of literature. Int J Angiol. 2008; 17:211–213.
8. Yoon JH, Han SS, Cha SS, Lee SJ. Color Doppler ultrasonography of a pancreatic arteriovenous malformation. J Ultrasound Med. 2005; 24:113–117.
9. Walter JF, Chuang VP, Bookstein JJ, Reuter SR, Cho KJ, Pulmano CM. Angiography of massive hemorrhage secondary to pancreatic diseases. Radiology. 1977; 124:337–342.
10. Chang S, Lim HK, Lee WJ, Choi D, Jang KT. Arteriovenous malformation of the pancreas in a patient with gastrointestinal bleeding: helical CT findings. Abdom Imaging. 2004; 29:259–262.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download