Abstract
We present a 53-year-old woman with a large chest wall mass in the interpectoral space, which was eventually confirmed as a lipogranuloma resulting from hydrogel implant rupture. Ultrasonography (US) showed reduced implant volume with surrounding peri-implant fluid collection, suggesting the possibility of implant rupture. A heterogeneously hypoechoic mass was found between the pectoralis major and minor muscles adjacent to the ruptured implant. On magnetic resonance imaging (MRI), there was a large mass in the left interpectoral space of the upper inner chest wall. The mass showed slightly high signal intensity (SI) on pre-contrast T1-weighted image (WI) with mixed iso and high SI on T2-WI. The signal of the mass was suppressed using the water suppression technique but not with the fat suppression technique on T2-WI. The mass showed diffuse enhancement upon contrast enhancement. The enhancing kinetics showed persistent enhancement pattern. US-guided core needle biopsy revealed a lipogranuloma and removal confirmed a ruptured PIP hydrogel implant.
Hydrogel implants became alternative fillers for breast implants after silicone implants were restricted in 1992 due to various local complications (1). Hydrogel implants contain hydrogel fillers, which are organic polymers of polysaccharide and water, such as hyaluronic acid fluid, hydroxypropyl cellulose (polysaccharide), and polyacrylamide (2). Initially, this implant was highly favored due to its improved radiolucency and biocompatibility, however, it was also banned in 2000 due to various complications such as ruptures, leakage of the osmotic hydrogel filler from the implant into the surrounding capsule without rupture (3), breast implant swelling or fluid collection due to osmotic instability (4) or fibrotic and inflammatory changes in adjacent tissues due to spilled implant materials (12). A few cases of foreign body reaction due to hydrogel leakage were reported (234).
To the best of our knowledge, there is no report of chest wall lipogranulomas caused by hydrogel implant rupture. We report the imaging and pathologic findings of chest wall lipogranuloma after hydrogel implant rupture that developed 17 years after implant insertion.
A 53-year-old female visited our institution due to a slow developing asymmetry of her left breast contour beginning 2 weeks earlier. She had a history of augmentation mammoplasty 17 years prior at another institution. She was unaware of the implant type. She had no previous cancer history. Physical examination revealed a large palpable lesion in the upper aspect of the left implant. There was no tenderness, redness, ulceration or skin color change in either breast.
On mammography, the right subpectoral implant appeared translucent but the left breast implant was not visible. Ultrasonography (US) showed the implants in the subpectoral space and the left implant appeared to have a reduced volume with a surrounding peri-implant fluid collection, suggesting the possibility of implant rupture. On the upper aspect of the implant, there was a large, heterogeneously hypoechoic mass between the pectoralis major and minor muscles (Fig. 1).
On magnetic resonance imaging (MRI), there was an 8 × 8 × 2 cm mass in the left interpectoral space of the upper inner chest wall. The mass showed slightly high signal intensity (SI) compared to the muscle in pre-contrast T1-weighted image (WI) and showed a mixture of iso and high SI on T2-WI. The signal of the mass was suppressed with the water suppression technique but not with the fat suppression technique on T2-WI. The mass showed diffuse enhancement upon contrast enhancement (Fig. 2). The enhancing kinetics showed persistent enhancement pattern.
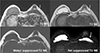
The left subpectoral implant was collapsed with a linguine sign, indicating a rupture. The SI of the implant was high on T2-WI and low on T1-WI. It was suppressed by the water suppressed technique but not by the fat suppression technique, which was compatible with hydrogel implants (Fig. 3).
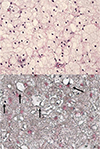
To exclude the possibility of implant-related tumorous condition, US-guided core needle biopsy was performed with a 14 gauge Tru-Cut needle. The Hematoxylin and Eosin staining of the core biopsy specimen consisted almost entirely of homogeneous foamy histiocytes with a background of focal sclerosis, suggestive of lipogranuloma. Neither demonstrable extracellular fatty tissue nor foreign body material were found. Intracellular fat lobules showed positive staining with Sudan black B stain, confirming the diagnosis of lipogranuloma (Fig. 4).
The implant was surgically removed and was found to be a Poly Implant Prosthesis (PIP) Hydrogel implant with a volume of 170 cc and a small hole in the shell. The mass was not excised during the surgery because the surgeon believed the pathologic nature would be safe and the patient was scheduled for follow up.
Lipogranuloma is a non-allergic foreign body reaction associated with exogenous or endogenous lipids, which can occur infrequently in the breast or axilla as a result of trauma or direct injection of cosmetic or reconstructive exogenous materials such as injectable mineral oils (paraffin) or liquid silicone (polydimethyl siloxane) (5). Non-mammary lipogranulomas have been reported as periorbital lipogranulomas after endoscopic sinus surgery (6). Sclerosing lipogranulomas have also been reported in male genitalia or in conjunctiva (7).
In our case, the lipogranuloma presented as a large mass-like lesion in the interpectoral space of the chest wall, the same anatomic compartment containing the ruptured implant. Previous imaging reports on spilled PIP hydrogel material in extracapsular rupture cases showed a migration of the PIP material to the chest wall, pectoralis muscle, and intercostal areas (2), which was similar to our case. Spilled hydrogel materials show the same SI as an implant on MRI without enhancement (2). Spilled PIP materials typically cause vigorous inflammation and fibrosis in adjacent structures such as capsules and breast tissues and a few cases resulted in granulomatous reactions with large numbers of foamy histiocytes with or without fat necrosis (234). These cases showed an enhancement along the capsule or the interface between the spilled hydrogel and adjacent tissue (2). But our case showed a diffuse enhancement of the chest wall mass, indicating that the entire chest wall mass was due to granuloma or an inflammatory lesion rather than the migration of spilled hydrogel material.
Patients with PIP hydrogel implants sometimes experience breast enlargement even without implant rupture due to swelling of the implant and fluid collection caused by osmotic instability, since hydrogel materials have the ability to swell in water without dissolving and to retain water within the structure (1234). For the same reason, extracapsular hydrogel can cause interstitial edema of adjacent breast tissue in MRI (1), but this could be distinguished from our case due to the lack of enhancement and infiltrative features on MRI. Histopathologically, spilled hydrogel materials show a weak foreign body reaction with interstitial edema and fibrosis of the breast tissue (1).
In our case, the lipogranuloma contained entirely homogeneous lipid-containing macrophages with focal sclerosis. We also observed a relatively weak foreign body reaction and a lack of extracellular fat vacuoles, which differed from previous reports of typical lipogranulomas (567) and might be the cause of the radiologic difference. Typical lipogranulomas show a signal decrease on fat-suppressed MR sequences due to free fat vacuoles in the extracellular space and heterogeneous enhancement (67). On the contrary, almost all tiny fat vacuoles in our case were located within the foamy macrophages, which might be the reason for the minimal signal decrease on fat suppressed MR sequence.
Other possible diagnoses for fat containing lesions of the breast are liponecrotic cysts or fat necrosis, which is a well-known complication of autologous fat injection in breasts (8910). Fat necrosis commonly show dystrophic calcifications or wall calcifications in mammography (8910), but lipogranulomas are not commonly associated with calcifications. Only one case of calcified lipogranuloma of the breast was reported to be caused by trauma with osseous metaplasia (5).
There is no data in the literature regarding the degradation potential of hydrogel implants or the potential toxicity of any resulting degradation products on the surrounding silicone shell or tissues. However, several reports suggested that the high rupture rate of hydrogel implants and the subsequent vigorous inflammation caused by spilled hydrogel material was due to the degradation potential and toxicity of hydrogel material (34). We suspect that the reason for lipogranuloma formation in our case may be endogenous fat degradation by spilled hydrogel material, resulting in foreign body reaction.
In summary, we reported a case of a large chest wall lipogranuloma caused by breast PIP hydrogel implant rupture, which was seen as an inflammatory mass without signal suppression by the fat suppression technique on MRI. Lipogranuloma can be considered as a rare complication of hydrogel implant rupture when a large non-calcified enhancing mass appears adjacent to the ruptured hydrogel implant.
Figures and Tables
Fig. 1
Transverse ultrasonography of left upper chest wall (a) and breast area (b). (a) Ultrasonography shows a 5-cm-sized, ill-defined heterogeneously echogenic mass (arrows) between pectoralis major (PM) and minor (Pm) muscle, superior to the implant. (b) The implant shows a reduced volume with peri-implant fluid collection (dotted arrows).
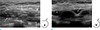
Fig. 2
Axial MRI of the breast. On MRI at the mass level, an 8 × 8 × 2 cm mass in the left interpectoral space of upper inner chest wall (arrows) shows slightly high signal intensity (SI) compared to the muscle in pre-contrast T1-weighted image (WI) and it shows a mixture of iso and high SI on T2-WI. The signal of the mass is suppressed by water suppressed T2-WI but not by fat suppressed T2-WI. The mass shows diffuse enhancement by contrast enhanced (CE) T1-WI.
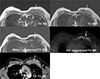
Fig. 3
On MRI at the implant level, the left subpectoral implant is collapsed with a linguine sign, representing a rupture (arrows). The SI of the implant is low on T1-WI and high in T2-WI and is suppressed by water suppressed T2-WI but not by fat suppressed T2-WI, which is compatible with hydrogel implants and not with silicone implants.
Acknowledgments
We thank the resident of pathology, Seokhwi Kim, for helping us obtain appropriate pathologic images.
References
1. Lee CJ, Park JH, Park IS, Lee SI. A case report of prefilled polysaccharide hydrogel breast implant rupture: clinical, MRI, and pathologic findings. Aesthetic Plast Surg. 2004; 28:401–404.
2. Choi JJ, Lee JH, Kang BJ, et al. Clinical and imaging characteristics of Polyimplant Prosthesis hydrogel breast implants. J Comput Assist Tomogr. 2010; 34:449–455.
3. Adams TS, Crook T, Cadier MA. A late complication following the insertion of hydrogel breast implants. J Plast Reconstr Aesthet Surg. 2007; 60:210–212.
4. O'Neill JK, Rigby H, Kenealy JM. Leakage and osmotic shifts in PIP Hydrogel implants. A case demonstrating increase and decrease of implant volume in the same patient. J Plast Reconstr Aesthet Surg. 2008; 61:1122–1123.
5. Lee HH, Park SH, Choi HY, Park HK. Lipogranuloma with osseous metaplasia in the breast that developed after "Bu-Hwang" oriental medicine treatment. Yonsei Med J. 2011; 52:373–376.
6. Yang BT, Liu YJ, Wang YZ, Wang XY, Wang ZC. CT and MR imaging findings of periorbital lipogranuloma developing after endoscopic sinus surgery. AJNR Am J Neuroradiol. 2012; 33:2140–2143.
7. Motoori K, Takano H, Ueda T, Ishihara M. Sclerosing lipogranuloma of male genitalia: CT and MR images. J Comput Assist Tomogr. 2002; 26:138–140.
8. Hyakusoku H, Ogawa R, Ono S, Ishii N, Hirakawa K. Complications after autologous fat injection to the breast. Plast Reconstr Surg. 2009; 123:360–370.
9. Veber M, Tourasse C, Toussoun G, Moutran M, Mojallal A, Delay E. Radiographic findings after breast augmentation by autologous fat transfer. Plast Reconstr Surg. 2011; 127:1289–1299.
10. Kim H, Yang EJ, Bang SI. Bilateral liponecrotic pseudocysts after breast augmentation by fat injection: a case report. Aesthetic Plast Surg. 2012; 36:359–362.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download