Abstract
Purpose
To investigate correlations of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor type 2 (HER2) statuses with magnetic resonance imaging (MRI) features and clinicohistological characteristics in patients with invasive lobular carcinoma (ILC).
Materials and Methods
Data from 64 histologically confirmed ILCs were analyzed retrospectively. Preoperative breast MRI was reviewed for morphology and dynamic contrast-enhanced kinetics of the tumor. Pathologic reports were reviewed for ER, PR, and HER2 positivity, tumor size, lymph node metastasis, and the number of metastatic lymph nodes. Furthermore, there was an investigation of the MRI features and clinicohistologic characteristics, according to the ER, PR, and HER2 statuses.
Results
A significant difference in MRI features and clinicohistological tumor characteristics were observed only in relation to PR status. Of the 64 ILCs, 10 (15.6%) were PR negative. PR negative cancers, compared with PR positive cancers, were more likely to present as non-mass enhancement (P = 0.027); have a significantly larger mean tumor size (5.00 ± 1.05 cm vs. 2.57 ± 0.21 cm, P = 0.021); and have significantly more metastatic lymph nodes (P = 0.010).
First described by Foote and Stewart in 1941, invasive lobular carcinoma (ILC) is the second most common histopathological subtype of breast cancer and is found in approximately 5-15% of patients. The incidence rate has steadily increased, especially among postmenopausal women, probably because of the use of hormone replacement therapy (1234).
Estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor type 2 (HER2) statuses of the tumor are important biomarkers for predicting prognoses and responses to therapy in patients with breast cancer (567).
Recently, correlations between magnetic resonance imaging (MRI) features and ER, PR, and HER2 statuses of breast cancer have been reported (891011); however, relatively little is known about ILC, despite its increasing incidence.
The aim of this study was to investigate associations between MRI features and ER, PR, and HER2 statuses in patients with ILC.
The study protocol was approved by the Institutional Review Board at the Gachon University of Medicine and Science, South Korea (GBIRB 2015-95). The requirement for informed consent of patients was waived because of the retrospective nature of this review.
A retrospective analysis was conducted of the medical records of patients with surgically confirmed ILC treated at the Gachon University of Medicine and Science between 2009 and 2014. We identified 71 ILC patients based on pathological data. Two patients who underwent excisional biopsy before breast MRI and six patients without preoperative breast MRI were excluded; hence, data from 63 patients were available. One patient had bilateral cancer; therefore, there were a total of 64 cancers from 64 affected breasts.
Further clinical information was also reviewed, including the patient age, symptoms or signs (tumor palpability and nipple discharge), and family history of breast cancer.
The MRI study was performed with patients in the prone position with 3.0 Tesla machines (Skyra and Verio, Siemens Medical Solutions, Erlangen, Germany) equipped with a breast coil. The imaging protocol consisted of a fat-suppressed T1-weighted, T2-weighted, axial or sagittal, 3-dimensional, gradient echo sequence, and dynamic contrast-enhanced (DCE) images. Imaging with the 3.0-T Skyra scanner covered both breasts with a minimum repetition time and echo time (4.79/1.83 ms for the dynamic images), a 10 flip angle, a 34 cm field of view, 1.5 mm section with no gap, a 512 × 512 matrix, and a scan time of 1-2 min. Imaging with the 3.0-T Verio scanner covered both breasts with a minimum repetition time and echo time (4.7/1.78 ms for the dynamic images), a 10 flip angle, a 35 cm field of view, 1.5 mm section with no gap, a 512 × 512 matrix, and a scan time of 1-2 min. For dynamic contrast enhancement, 0.1 mmol/kg body weight gadobutrol (Gadovist; Schering AG, Berlin, Germany) was injected, followed by a 20-mL saline flush. After the examination, two subtraction images were made automatically on a pixel-by-pixel basis; the unenhanced images were subtracted from the early post-contrast images (standard subtraction), and the last post-contrast image was subtracted from the early post-contrast images (reverse subtraction). The reformatted images with a maximum intensity projection were then created from the standard and reverse subtraction images. The breast MR images were interpreted by one of two faculty radiologists present at that time.
The images were interpreted independently by two breast radiologists (Y.E.Y., N.S.Y., with 6-7 years of experience in breast imaging) who were blinded to the clinical and radiologic information of all patients. The image manipulations did not involve computer-aided detection. Interpretations were performed by consensus review to resolve differences after their review of images.
The morphology, DCE kinetics, and multiplicity were analyzed based on the American College of Radiology Breast Imaging Reporting and Data System MRI lexicon, 2nd edition (12). The lesions were classified as mass or non-mass enhancement. The masses were evaluated for shape (oval, round, irregular), margin (circumscribed, irregular, spiculated), and internal enhancement characteristics (homogeneous, heterogeneous, rim enhancement). Furthermore, there were also analyses of the lesion distribution (focal, linear, segmental, regional, multiple regions, diffuse) and internal enhancement pattern (homogeneous, heterogeneous, clumped, clustered ring) of non-mass enhancement lesions. The DCE kinetic time-intensity curve was evaluated based on initial and delayed phases. The initial phase was described as slow, medium, and fast. The delayed phase was categorized into three types: type 1-persistent, type 2-plateau, or type 3-washout. Multiplicity was defined as the presence of more than one unconnected lesion in a breast. When there were multiple lesions in one breast, only the largest lesion was analyzed.
We reviewed the pathological reports for the following parameters: tumor size, multiplicity, nodal involvement, number of metastatic lymph nodes, and ER, PR, and HER2 statuses. The positive ER and PR statuses were defined by an Allred score greater than or equal to 3, based on immunohistochemical staining. The HER2 status was considered positive if the immunohistochemical stain results indicated an Allred score of 3+ or 2+, with confirmation of HER2 gene amplification by silver in situ hybridization.
Statistical analysis of the patients' ages and tumor sizes was performed using the Student t-test and Mann-Whitney U test, respectively. Multiplicity, lymph node metastasis, and the number of lymph node metastases were compared using the chi-square test. Differences in MRI features according to ER, PR, and HER2 statuses were investigated using the chi-square test or the Fisher exact test. All statistical analyses were performed using SPSS software version 19.0 (SPSS Inc., Chicago, IL, USA); and a P value < 0.05 was considered statistically significant.
The mean (± standard deviation) age of the patients was 49.78 (± 1.02) years. Of the 64 ILCs, 59 (92.2%), 54 (84.4%), and 49 (76.6%) were ER positive, PR positive, and HER2 negative, respectively. Significant differences in clinicohistological features correlated only with PR status. A comparison of the clinicohistological characteristics between the PR positive and PR negative cancers is shown in Table 1. PR negativity was associated with larger tumor sizes (P = 0.046). A total of 20 PR positive patients (37.0%) and 6 PR negative patients (60%) had lymph node metastases, which was a statistically insignificant difference (P = 0.157); however, the number of positive metastatic lymph nodes was significantly different between PR positive and PR negative patients (P = 0.010). There was no correlation between ER or HER2 statuses and clinicohistological features in ILCs (P > 0.05).
The mean tumor size on MRI was 2.96 ± 0.26 cm. Tumor measurements determined by MRI were not significantly different from those recorded in the pathological reports (3.48 ± 0.33 cm). A significant difference in MRI features was only noted according to the PR status of the tumor. Table 2 summarizes the comparison of analyzed MRI features from 54 PR positive cancers (the data are from 53 patients; one had bilateral cancer) and 10 PR negative cancers. A total of 49 of 54 PR positive cancers (90.7%) showed mass lesions (Fig. 1) and five (9.3%) showed non-mass enhancement. A total of six of the 10 PR negative cancers (60%) showed mass lesions and four (40%) showed non-mass enhancement (Fig. 2). Therefore, PR negative cancers were more likely to show non-mass enhancement (4/10 vs. 5/54, P = 0.027). Of the non-mass enhancement cancers, PR negative cancer was more likely to show clustered ring internal enhancement pattern on MRI than PR positive cancer (P = 0.056), but the difference was not statistically significant. There was no significant difference in MRI features according to ER or HER2 statuses (Table 3).
In this study, we examined the association of hormonal receptor status with MRI features and clinicohistological characteristics of ILC. We found that PR negativity was associated with non-mass enhancement pattern on MRI and larger tumor sizes. In addition, PR negative ILC had significantly more disease positive lymph nodes than PR positive ILC.
Breast cancers with different ER, PR, and HER2 statuses have different prognoses and respond differently to endocrine therapy (5), radiation therapy (6), and chemotherapy (7). Previous analyses investigated whether information on tumor PR status can provide additional value to ER status and improve the prediction of benefit of endocrine treatment in breast cancer patients (1314). Reports suggest that a negative PR status is an important contributor to the relapse risk in the early (15) and late (16) periods of time after diagnoses. PR negative patients have a shorter disease-free interval than PR positive patients, and PR status is a more valuable predictor of the disease-free interval than ER status (17).
In a recent study by Knopfelmacher et al. (18), they correlated histopathologic features with the biology of ductal carcinoma in situ, and found that dense chronic inflammation surrounding ductal carcinoma in situ were more common in PR negative tumors. This result supported our finding that PR negative cancer had more non-mass enhancement pattern on MRI.
PR negativity also significantly correlates with a higher grade of cell differentiation and nuclear polymorphism, higher rate of mitosis, and lower tubular differentiation (19). In addition, there is a highly significant negative correlation between PR status and CD 34, CD 105 counts that reflect angiogenesis (20); and PR negative cancers exhibit significant inflammatory lymphocytic infiltrate in the tumor stroma (21). These pathological features of PR negative cancer may provide a basis for presenting larger tumor sizes on MRI.
Reports on the correlation between ER, PR, and HER2 statuses and imaging features are rare. A previous study including 21 invasive ductal carcinomas (IDCs) and 4 ILCs, reported that PR negative breast cancer had significantly larger tumor sizes and more non-mass type lesions on MRI; however, exclusion of the 4 ILCs from the analysis revealed no significant correlation between the PR status of the IDC cases and differences in MRI features (22). ER negative breast cancers are also reported to be more aggressive, with larger tumor sizes and more non-mass enhancement pattern on MRI; however, this group only included cases of IDC (23). In this study, we found that tumor PR negativity was associated with larger tumor sizes and presented with non-mass enhancement pattern on MRI in patients with ILC. However, there was no significant difference in MRI features and histologic features according to ER expression. A possible explanation for this apparent contradiction is that the proportion of ER positive cancers in ILC was higher than that in IDC. Actually, a total of 59 out of 64 ILCs (92.2%) were ER positive in this study.
Axillary lymph node involvement is one of the most important prognostic factors in patients with invasive breast cancer, whereas patients with an increasing number of metastatic lymph nodes have a poorer prognosis (24). Previous studies showed that both ER and PR status are not reliable predictors of lymph node metastasis (2324). In this study, lymph node metastasis was similar between the PR negative and PR positive ILC; however, there was a significant increase in the number of the positive nodes in the PR negative group compared with that in the PR positive group.
There are several limitations in this study. First, this was a retrospective study design and the number of enrolled patients may be too small to reach a definitive conclusion. Furthermore, this study was limited in the usage of pathologic data, such as histologic grade, lymphovascular invasion, and Ki-67, because those were not recorded in some pathologic reports. A large scale prospective study will provide results of more specific findings in MRI features.
In conclusion, PR negative ILC presented more frequently as non-mass enhancement on MRI, with larger tumor sizes. In addition, the number of metastatic lymph nodes was significantly increased in PR negative cancer. These features might be associated with a higher grade of cell differentiation, more angiogenesis or lymphocytic stroma, or both. Based on the results, we suggest that PR status plays an important role in determining MRI features and clinicohistological characteristics for ILC. Furthermore, knowledge of the PR expression status could help in surgical planning and management.
Figures and Tables
Fig. 1
A 40-year-old woman with PR positive ILC. (a) Axial T2-weighted image with fat suppression shows a hyperintense lesion in the right breast (arrow). (b) Axial contrast-enhanced MR image in early dynamic phase shows a heterogeneously enhanced irregular mass (arrow). (c) Kinetic curve of dynamic MRI shows initial fast and delayed washout enhancement pattern.

Fig. 2
A 43-year-old woman with PR negative ILC. (a) Axial T2-weighted image shows a slightly hyperintense lesion in the right breast (arrow). (b) Axial contrast-enhanced MR image in early dynamic phase shows segmental heterogeneous internal enhancement pattern of non-mass enhancement (arrow). (c) The MIP image also demonstrates a non-mass enhancement lesion (arrows).
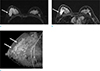
Table 1
Comparison of Clinicohistological Characteristics between PR Positive and PR Negative ILCs

Table 2
MR Imaging Features of PR Positive and PR Negative ILCs

Table 3
The Relationship between MRI Features and ER and HER2 Statuses

References
1. Mann RM, Hoogeveen YL, Blickman JG, Boetes C. MRI compared to conventional diagnostic work-up in the detection and evaluation of invasive lobular carcinoma of the breast: a review of existing literature. Breast Cancer Res Treat. 2008; 107:1–14.
2. Li CI, Anderson BO, Daling JR, Moe RE. Trends in incidence rates of invasive lobular and ductal breast carcinoma. JAMA. 2003; 289:1421–1424.
3. Li CI, Anderson BO, Porter P, Holt SK, Daling JR, Moe RE. Changing incidence rate of invasive lobular breast carcinoma among older women. Cancer. 2000; 88:2561–2569.
4. Arpino G, Bardou VJ, Clark GM, Elledge RM. Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome. Breast Cancer Res. 2004; 6:R149–R156.
5. Rastelli F, Crispino S. Factors predictive of response to hormone therapy in breast cancer. Tumori. 2008; 94:370–383.
6. Kyndi M, Sorensen FB, Knudsen H, et al. Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: the Danish Breast Cancer Cooperative Group. J Clin Oncol. 2008; 26:1419–1426.
7. Fernandez-Morales LA, Segui MA, Andreu X, et al. Analysis of the pathologic response to primary chemotherapy in patients with locally advanced breast cancer grouped according to estrogen receptor, progesterone receptor, and HER2 status. Clin Breast Cancer. 2007; 7:559–564.
8. Lee SH, Cho N, Kim SJ, et al. Correlation between high resolution dynamic MR features and prognostic factors in breast cancer. Korean J Radiol. 2008; 9:10–18.
9. Montemurro F, Martincich L, Sarotto I, et al. Relationship between DCE-MRI morphological and functional features and histopathological characteristics of breast cancer. Eur Radiol. 2007; 17:1490–1497.
10. Szabo BK, Aspelin P, Kristoffersen Wiberg M, Tot T, Bone B. Invasive breast cancer: correlation of dynamic MR features with prognostic factors. Eur Radiol. 2003; 13:2425–2435.
11. Teifke A, Behr O, Schmidt M, et al. Dynamic MR imaging of breast lesions: correlation with microvessel distribution pattern and histologic characteristics of prognosis. Radiology. 2006; 239:351–360.
12. Morris EA, Comstock CE, Lee CH, et al. ACR BI-RADS® Magnetic Resonance Imaging. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology;2013.
13. Bardou VJ, Arpino G, Elledge RM, Osborne CK, Clark GM. Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J Clin Oncol. 2003; 21:1973–1979.
14. Anderson H, Hills M, Zabaglo L, et al. Relationship between estrogen receptor, progesterone receptor, HER-2 and Ki67 expression and efficacy of aromatase inhibitors in advanced breast cancer. Ann Oncol. 2011; 22:1770–1776.
15. Chen L, Romond E, Chokshi S, et al. A prognostic model of early breast cancer relapse after standard adjuvant therapy and comparison with metastatic disease on initial presentation. Breast Cancer Res Treat. 2012; 136:565–572.
16. Nishimura R, Osako T, Nishiyama Y, et al. Evaluation of factors related to late recurrence--later than 10 years after the initial treatment--in primary breast cancer. Oncology. 2013; 85:100–110.
17. Gelbfish GA, Davidson AL, Kopel S, et al. Relationship of estrogen and progesterone receptors to prognosis in breast cancer. Ann Surg. 1988; 207:75–79.
18. Knopfelmacher A, Fox J, Lo Y, Shapiro N, Fineberg S. Correlation of histopathologic features of ductal carcinoma in situ of the breast with the oncotype DX DCIS score. Mod Pathol. 2015; [Epub ahead of print].
19. Stierer M, Rosen H, Weber R, Hanak H, Spona J, Tuchler H. Immunohistochemical and biochemical measurement of estrogen and progesterone receptors in primary breast cancer. Correlation of histopathology and prognostic factors. Ann Surg. 1993; 218:13–21.
20. Dhakal HP, Naume B, Synnestvedt M, et al. Vascularization in primary breast carcinomas: its prognostic significance and relationship with tumor cell dissemination. Clin Cancer Res. 2008; 14:2341–2350.
21. Park SY, Kim HM, Koo JS. Differential expression of cancer-associated fibroblast-related proteins according to molecular subtype and stromal histology in breast cancer. Breast Cancer Res Treat. 2015; 149:727–741.
22. Chen JH, Nalcioglu O, Su MY. MR imaging features of invasive breast cancer correlated with hormonal receptors: does progesterone receptor matter? Ann Oncol. 2008; 19:1024–1026.
23. Chen JH, Baek HM, Nalcioglu O, Su MY. Estrogen receptor and breast MR imaging features: a correlation study. J Magn Reson Imaging. 2008; 27:825–833.
24. Aitken E, Osman M. Factors affecting nodal status in invasive breast cancer: a retrospective analysis of 623 patients. Breast J. 2010; 16:271–278.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download