Abstract
Purpose
Catheter associated urinary tract infection (CAUTI) frequently occurs in the patients with an indwelling Foley catheter, and it can cause serious morbidity or mortality. However, there have been no reports about quorum sensing mechanisms in indwelling Foley catheter. It's our purpose to find out the quorum sensing mechanisms of isolated bacteria from biofilm in Foley catheters.
Materials and Methods
Silicone Foley catheters were placed in 90 patients with neurogenic bladders. At the 3rd, 5th, 7th, 14th and 30th day after the catheters were placed, the catheters were removed and the biofilm formations were evaluated by routine culture and microscopy. The ygaG gene, which was reported to be an autoinducer synthase gene was carried out cloning in E. coli. The quantity of the mRNA expression of the ygaG gene was analyzed according to the time by competitive reverse transcription polymerase chain reaction (RT-PCR).
Results
289 different types of bacteria were isolated by cultivation. The most common species were Pseudomonas, Klebsiella, Serratia, Proteus and Escherichia species. The autoinducer synthase gene, such as the ygaG gene for Escherichia coli, was detected by RT-PCR. On competitive RT-PCR of the ygaG gene, the mRNA expression was 3.77×109copies/µl at the 3rd day, 5.94×107copies/µl at the 5th day, 8.07×107copies/µl at the 7th day, 2.51×106copies/µl at the 14th day and 6.81×108copies/µl at the 30th day. Therefore, the expression of the autoinducer synthase gene was observed at the early insertion period and it was then maintained.
Figures and Tables
Fig. 1
(A) The profile of bacteria according to time (in an aerobic condition). (B) The profile of bacteria according to time (in an anaerobic condition).

Fig. 2
Amplification of the ygaG gene used for RNA sampling by reverse transcription-polymerase chain reaction (RT-PCR). Lane M is the molecular size marker. Lanes 1 and 2 of panels A and B are products of the ygaG gene, which are isolated from bacteria in each of the 3, 5, 7 and 14 days catheters, as the template and ygaG primer. Lane 1 of panel C is the products of the ygaG gene, which is isolated from bacteria in the 30 days catheter.
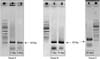
Fig. 3
Ethidium bromide stained gel used to generate the equation for quantitative competitive reverse transcription-polymerase chain reaction (RT-PCR) for quantitative analysis of the RNA sample according to the insertion time of the catheter. Lane M is a 1kb DNA ladder. The cDNA of the total RNA from bacteria isolated from a catheter, according to the insertion time, is used as template, and the PCR reactions of lanes 1-9 of panel A contained 10-1, 10-2, 10-3, 10-3.5, 10-3.7, 10-4, 10-3.7, 10-4.1, 10-4.2, respectively, of the competitor cDNA. The PCR reactions of lane 1-6 of panel B contained 10-5, 10-5.3, 10-5.7, 10-2, 10-2.5, 10-3, respectively, of the competitor cDNA.
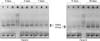
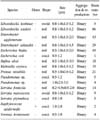
Table 1
Oligonucleotide primers for the ygaG and the competitor used for polymerase chain reaction (PCR) amplification and sequencing

References
1. Rumbaugh KP, Griswold JA, Hamood AN. The role of quorum sensing in the in vivo virulence of Pseudomonas aeruginosa. Microbes Infect. 2000. 2:1721–1731.
2. Nickel JC, Grant SK, Costerton JW. Catheter-associated bacteriuria. An experimental study. Urology. 1985. 26:369–375.
3. Parsek MR, Greenberg EP. Acyl-homoserine lactone quorum sensing in gram-negative bacteria: a signaling mechanism involved in associations with higher organisms. Proc Natl Acad Sci USA. 2000. 97:8789–8793.
4. Bauer WD, Robinson JB. Disruption of bacterial quorum sensing by other organisms. Curr Opin Biotechnol. 2002. 13:234–237.
5. DeLisa MP, Valdes JJ, Bentley WE. Mapping stress-induced changes in autoinducer AI-2 production in chemostat-cultivated Escherichia coli K-12. J Bacteriol. 2001. 183:2918–2928.
6. Donabedian H. Quorum sensing and its relevance to infectious diseases. J Infect. 2003. 46:207–214.
7. Hassett DJ, Cuppoletti J, Trapnell B, Lymar SV, Rowe JJ, Yoon SS, et al. Anaerobic metabolism and quorum sensing by Pseudomonas aeruginosa biofilms in chronically infected cystic fibrosis airways: rethinking antibiotic treatment strategies and drug targets. Adv Drug Deliv Rev. 2002. 54:1425–1443.
8. Kjelleberg S, Molin S. Is there a role for quorum sensing signals in bacterial biofilms? Curr Opin Microbiol. 2002. 5:254–258.
9. Tenke P, Jackel M, Nagy E. Prevention and treatment of catheter-associated infections: myth or reality. EAU update Series. 2004. 2:106–115.
10. Thompson LS, Webb JS, Rice SA, Kjelleberg S. The alternative sigma factor RpoN regulates the quorum sensing gene rhlI in Pseudomonas aeruginosa. FEMS Microbiol Lett. 2003. 220:187–195.
11. Kim DI, Lee SJ, Cho YH. Norfloxacin hydrogel coated urethral catheters for prevention of catheter associated urinary tract infection in the rabbit. Korean J Urol. 2002. 43:980–986.
12. Kumon H, Hashimoto H, Nishimura M, Monden K, Ono N. Catheter-associated urinary tract infections: impact of catheter materials on their management. Int J Antimicrob Agents. 2001. 17:311–316.
13. Merle V, Germain JM, Bugel H, Nouvellon M, Lemeland JF, Czernichow P, et al. Nosocomial urinary tract infections in urologic patients: assessment of a prospective surveillance program including 10,000 patients. Eur Urol. 2002. 41:483–489.
14. Tambyah PA. Catheter-associated urinary tract infections: diagnosis and prophylaxis. Int J Antimicrob Agents. 2004. 24:Suppl 1. S44–S48.
15. Lee JH, Ju YM, Kim DM. Platelet adhesion onto segmented polyurethane film surfaces modified by addition and crosslinking of PEO-containing block copolymers. Biomaterials. 2000. 21:683–691.
16. Tal S, Guller V, Levi S, Bardenstein R, Berger D, Gurevich I, et al. Profile and prognosis of febrile elderly patients with bacteremic urinary tract infection. J Infect. 2005. 50:296–305.
17. Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002. 15:167–193.
18. Reissbrodt R, Rienaecker I, Romanova JM, Freestone PP, Haigh RD, Lyte M, et al. Resuscitation of Salmonella enterica serovar typhimurium and enterohemorrhagic Escherichia coli from the viable but nonculturable state by heat-stable enterobacterial autoinducer. Appl Environ Microbiol. 2002. 68:4788–4794.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download