Abstract
Purpose
Molecular magnetic resonance (MR) imaging techniques using superparamagnetic iron oxide nanocrystals (SPIO) have been developed for noninvasively monitoring stem cells. This study was performed to investigate if the presence of transplanted human mesenchymal stem cells in the liver, kidney, bladder and penile cavernosum can be evaluated noninvasively with using molecular MR imaging.
Materials and Methods
SPIO (Feridex; AMI, Cambridge, USA) were transferred to the human mesenchymal stem cells (hMSCs) using Gene-PORTER. The labeling viability, efficiency and differentiation of the SPIO transferred hMSCs were examined with Tripan blue, Von Kossa, alkaline phosphatase, toluidine blue, oil red O and Prussian blue staining. The SPIO labelled hMSCs were transplanted to the liver, kidney, bladder and penile cavernosum of rats, and the MR images were examined in vitro or in vivo using 1.5 T MR.
Results
The viability and efficiency of the SPIO transferred hMSCs were good. Osteogenic, chondrogenic or adipogenic differentiation from the SPIO transferred hMSCs was observed. A decrease of the MR signal intensity of the SPIO transferred hMSCs with using GenePORTER was found in vitro. A decrease of the MR signal intensity was found at concentrations that were more than 1×105 hMSCs in vitro. The MR signal intensity at the areas of the SPIO transferred hMSCs decreased in the liver, kidney, bladder and penile cavernosum. The intracellular SPIOs were confirmed in the SPIO labelled hMSCs that were transplanted in the liver, kidney, bladder and penile cavernosum with Prussian blue staining.
Figures and Tables
Fig. 1
MR image of the SPIO labelled hMSCs in the liver. (A) Liver before SPIO labelled hMSCs transplantation. (B) The areas of decreased MR signal intensity in the liver are diffusely distributed 1 day after SPIO labelled hMSCs transplantation. The arrow shows the decrease of MR signal intensity. (C) Prussian blue stain (×200). The arrow shows the presence of SPIO labelled hMSCs. MR: magnetic resonance, SPIO: superparamagnetic iron oxide, hMSCs: human mesenchymal stem cells. The arrow shows the decrease of the MR signal intensity.

Fig. 2
MR image of the SPIO labelled hMSCs in the kidney. (A) Kidney before SPIO labelled hMSCs transplantation. (B) The areas of decreased MR signal intensity in the kidney are confined locally 1 day after SPIO labeled hMSCs transplantation. The arrow shows the decrease of MR signal intensity. (C) Prussian blue stain (×200). The arrow shows the presence of SPIO labelled hMSCs. MR: magnetic resonance, SPIO: superparamagnetic iron oxide, hMSCs: human mesenchymal stem cells. The arrow shows the decrease of MR signal intensity.
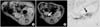
Fig. 3
MR image of the SPIO labelled hMSCs in the bladder. (A) Bladder wall before SPIO labelled hMSCs transplantation. (B) The areas of decreased MR signal intensity in the bladder are confined locally 1 days after SPIO labelled hMSCs transplantation. The arrow shows the decrease of the MR signal intensity. (C) Prussian blue stain (×200). The arrow shows the presence of SPIO labelled hMSCs. MR: magnetic resonance, SPIO: superparamagnetic iron oxide, hMSCs: human mesenchymal stem cells. The arrow shows the decrease of MR signal intensity.
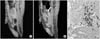
Fig. 4
MR image of the SPIO labelled hMSCs in the penis. (A) Bladder wall before SPIO labelled hMSCs transplantation. (B) The areas of decreased MR signal intensity in the penis are confined locally 1 day after SPIO labelled hMSCs transplantation. Arrow shows the decrease of MR signal intensity. (C) Prussian blue stain (×200). The arrow shows the presence of SPIO labelled hMSCs. MR: magnetic resonance, SPIO: superparamagnetic iron oxide.
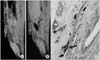
References
1. Ittrich H, Lange C, Dahnke H, Zander AR, Adam G, Nolte-Ernsting C. Labeling of mesenchymal stem cells with different superparamagnetic particles of iron oxide and detectability with MRI at 3T. Rofo. 2005. 177:1151–1163.
2. Matuszewski L, Persigehl T, Wall A, Schwindt W, Tombach B, Fobker M, et al. Cell tagging with clinically approved iron oxides: feasibility and effect of lipofection, particle size, and surface coating on labeling efficiency. Radiology. 2005. 235:155–161.
3. Unger EC. How can superparamagnetic iron oxides be used to monitor disease and treatment? Radiology. 2003. 229:615–616.
4. Tang YL, Zhao Q, Zhang YC, Cheng L, Liu M, Shi J, et al. Autologous mesenchymal stem cell transplantation induce VEGF and neovascularization in ischemic myocardium. Regul Pept. 2004. 117:3–10.
5. Nagaya N, Kangawa K, Itoh T, Iwase T, Murakami S, Miyahara Y, et al. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005. 112:1128–1135.
6. Park SK, Won JH, Park SJ, Chung NG, Jeong DC, Kim CK, et al. The role of mesenchymal stem cells in hematopoietic stem cell transplantation. Korean J Med. 2003. 65:277–288.
7. Arbab AS, Yocum GT, Wilson LB, Parwana A, Jordan EK, Kalish H, et al. Comparison of transfection agents in forming complexes with ferumoxides, cell labeling efficiency, and cellular viability. Mol Imaging. 2004. 3:24–32.
8. Djurovic S, Iversen N, Jeansson S, Hoover F, Christensen G. Comparison of nonviral transfection and adeno-associated viral transduction on cardiomyocytes. Mol Biotechnol. 2004. 28:21–32.
9. Kostura L, Kraitchman DL, Mackay AM, Pittenger MF, Bulte JW. Feridex labeling of mesenchymal stem cells inhibits chondrogenesis but not adipogenesis or osteogenesis. NMR Biomed. 2004. 17:513–517.
10. Nishida M, Higuchi H, Kobayashi Y, Takagishi K. Hitological and biochemical changes of experimental meniscus tear in the dog knee. J Orthop Sci. 2005. 10:406–413.
11. Yang M, Ma QJ, Dang GT, Ma K, Chen P, Zhou CY. In vitro and in vivo induction of bone formation based on ex vivo gene therapy using rat adipose-derived adult stem cells expressing BMP-7. Cytotherapy. 2005. 7:273–281.
12. Kang X, Xie Y, Kniss DA. Adipose tissue model using three-dimensional cultivation of preadipocytes seeded onto fibrous polymer scaffolds. Tissue Eng. 2005. 11:458–468.
13. Oweida AJ, Dunn EA, Foster PJ. Cellular imaging at 1.5T: detecting cells in neuroinflammation using active labeling with superparamagnetic iron oxide. Mol Imaging. 2004. 3:85–89.
14. Fleige G, Nolte C, Synowitz M, Seeberger F, Kettenmann H, Zimmer C. Magnetic labeling of activated microglia in experimental gliomas. Neoplasia. 2001. 3:489–499.
15. Bremer C, Allkemper T, Baerming J, Reimer P. RES-specific imaging of the liver and spleen with iron oxide particles designed for blood pool MR-angiography. J Magn Reson Imaging. 1999. 10:461–467.
16. Lewin M, Carlesso N, Tung CH, Tanf XW, Cory D, Scadden DT, et al. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat Biotechnol. 2000. 18:410–414.
17. Kraitchman DL, Heldman AW, Atalar E, Adamo LC, Martin BJ, Pittenger MF, et al. In vivo magnetic resonance image of mesenchymal stem cells in myocardial infarction. Circulation. 2003. 107:2290–2293.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download