Abstract
Purpose
Interleukin-6 (IL-6) can stimulate a variety of tumors including prostatic carcinoma. Research has recently shown that IL-6 may act to stimulate the progression of prostatic cancer. IL-6 is elevated in the sera of patients with metastatic prostatic cancer and it has been shown to be a candidate marker of disease activity. To date, little work has been performed to characterize the nature of granulocyte macrophage colony-stimulating factor (GM-CSF) and the expression of IL-6. The aim of this study is to evaluate the effects of GM-CSF on the expression of IL-6 in PC-3 cells.
Materials and Methods
The bone-derived PC-3 cell line was used in this study. Reverse transcription polymerase chain reaction (RT-PCR) was performed to detect the GM-CSF and also the IL-6 mRNA expression. The IL-6 protein was measured by enzyme-linked immunosorbent assay (ELISA) after treatments with the hGM-CSF.
Results
hGM-CSF was expressed in the PC-3 cell line. Our data indicated that the IL-6 mRNA expression was not increased at 4, 8 and 12 hours by the hGM-CSF in comparison to the control group, but it was slightly increased at 24 and 48 hours. The expression of IL-6 protein was increased at 4, 8, 12, 24 and 48 hours after hGM-CSF treatment, in comparison with the control group.
Conclusions
The IL-6 mRNA expression was slightly increased by hGM-CSF at 24 and 48 hours in comparison to the control group. Yet the IL-6 protein expression increased before the IL-6 mRNA expression. Therefore, hGM-CSF may modulate the post-transcription pathway of the IL-6 expression in prostate carcinoma cells. Our data suggest that GM-CSF may have a possible IL-6 mediated pathophysiologic role in prostate cancer.
Figures and Tables
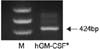 | Fig. 1hGM-CSF* expression in PC-3. *hGM-CSF: human granulocyte macrophage colony-stimulating factor. |
 | Fig. 2Time course of the IL-6mRNA expression in PC-3 cells. The PC-3 cells were treated with hGM-CSF for the indicated times. The IL-6mRNA expression is not increased at 4, 8 and 12 hours, but the IL-6mRNA expression is increased at 24 and 48 hours. *IL-6: interleukin-6, †GAPDH: glyceraldehyde-3-phosphate dehydrogenase. |
 | Fig. 3The averages of the interleukin-6 (IL-6) concentrations (ng/ml) and their standard deviation for the incubation periods as determined by ELISA. The IL-6 protein expression is significantly increased at 4, 8, 12, 24 and 48 hours after hGM-CSF treatment compared with the control group. *: p<0.05. |
References
1. Korea Statistical Information System. National Statistical office. Republic of Korea. Available from: URL: http://kosis.nso.go.kr.
2. Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol. 1993. 54:1–78.
3. Tan D, Wu X, Hou M, Lee SO, Lou W, Wang J, et al. Interleukin-6 polymorphism is associated with more aggressive prostate cancer. J Urol. 2005. 174:753–756.
4. Chung TD, Yu JJ, Spiotto MT, Bartkowski M, Simons JW. Characterization of the role of IL-6 in the progression of prostate cancer. Prostate. 1999. 38:199–207.
5. Offner FA, Obrist P, Stadlmann S, Feichtinger H, Klingler P, Herold M, et al. IL-6 secretion by human peritoneal mesothelial and ovarian cancer cells. Cytokine. 1995. 7:542–547.
6. Lee SO, Lou W, Hou M, de Miguel F, Gerber L, Gao AC. Interleukin-6 promotes androgen-independent growth in LNCaP human prostate cancer cells. Clin Cancer Res. 2003. 9:370–376.
7. Nicola NA. Hemopoietic growth factors and their interactions with specific receptors. J Cell Physiol. 1987. Suppl 5. 9–14.
8. Horwood NJ, Udagawa N, Elliott J, Grail D, Okamura H, Kurimoto M, et al. Interleukin 18 inhibits osteoclast formation via T cell production of granulocyte macrophage colony stimulating factor. J Clin Invest. 1998. 101:595–603.
9. Guillaume T, Sekhavat M, Rubinstein DB, Hamdan O, Symann ML. Transcription of genes encoding granulocyte-macrophage colony-stimulating factor, interleukin 3, and interleukin 6 receptors and lack of proliferative response to exogenous cytokines in nonhematopoietic human malignant cell lines. Cancer Res. 1993. 53:3139–3144.
10. Savarese DM, Valinski H, Quesenberry P, Savarese T. Expression and function of colony-stimulating factors and their receptors in human prostate carcinoma cell lines. Prostate. 1998. 34:80–91.
11. Rivas CI, Vera JC, Delgado-Lopez F, Heaney ML, Guaiquil VH, Zhang RH, et al. Expression of granulocyte-macrophage colony-stimulating factor receptors in human prostate cancer. Blood. 1998. 91:1037–1043.
12. Kim TH, Kim YS, Myung SC, Lee DH, Won EH, Lee SY. The prostaglandin E receptor agonists increase granulocyte macrophage colony-stimulating factor in prostate cancer cells. Korean J Urol. 2004. 45:1272–1278.
13. Lang SH, Miller WR, Duncan W, Habib FK. Production and response of human prostate cancer cell lines to granulocyte macrophage-colony stimulating factor. Int J Cancer. 1994. 59:235–241.
14. Rokhlin OW, Griebling TL, Karassina NV, Raines MA, Cohen MB. Human prostate carcinoma cell lines secrete GM-CSF and express GM-CSF-receptor on their cell surface. Anticancer Res. 1996. 16:557–563.
15. Culig Z. Interleukin-6 polymorphism: expression and pleiotropic regulation in human prostate cancer. J Urol. 2005. 174:417.
16. Nakashima J, Tachibana M, Horiguchi Y, Oya M, Ohigashi T, Asakura H, et al. Serum interleukin 6 as a prognostic factor in patients with prostate cancer. Clin Cancer Res. 2000. 6:2702–2706.
17. Drachenberg DE, Elgamal AA, Rowbotham R, Peterson M, Murphy GP. Circulating levels of interleukin-6 in patients with hormone refractory prostate cancer. Prostate. 1999. 41:127–133.
18. Siegall CB, Schwab G, Nordan RP, FitzGerald DJ, Pastan I. Expression of the interleukin-6 receptor and interleukin-6 in prostate carcinoma cells. Cancer Res. 1990. 50:7786–7788.
19. Okamoto M, Lee C, Oyasu R. Interleukin-6 as a paracrine and autocrine growth factor in human prostatic carcinoma cells in vitro. Cancer Res. 1997. 57:141–146.
20. Giri D, Ozen M, Ittmann M. Interleukin-6 is an autocrine growth factor in human prostate cancer. Am J Pathol. 2001. 139:2159–2165.
21. Charyulu VI, Lopez DM. Elevated GM-CSF levels in tumor bearing mice upregulate IL-6 production by B cells via a mechanism independent of TNF-α. Int J Oncol. 2000. 16:161–167.
22. Adler HL, McCurdy MA, Kattan MW, Timme TL, Scardino PT, Thompson TC. Elevated levels of circulating interleukin-6 and transforming growth factor-beta1 in patients with metastatic prostate carcinoma. J Urol. 1999. 161:182–187.
23. Twillie DA, Eisenberger MA, Carducci MA, Hseih WS, Kim WY, Simons JW. Interleukin-6: a candidate mediator of human prostate cancer morbidity. Urology. 1995. 45:542–549.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download