Abstract
Purpose:
The purpose of this study was to evaluate the validity of the cutoff size for a localized renal cell carcinoma (RCC) by assessing the survival of RCC patients according to a series of alternative size cutoff values.
Materials and Methods:
The outcomes of 147 patients with localized RCC, treated by radical nephrectomy at our institution, between 1984 and 2004, were retrospectively evaluated. The mean follow-up period was 54.9±32.5 months. The survival of patients with tumors smaller than a specified size cutoff was compared with that of tumors larger than the cutoff, and the most discriminating cutoff identified.
Results:
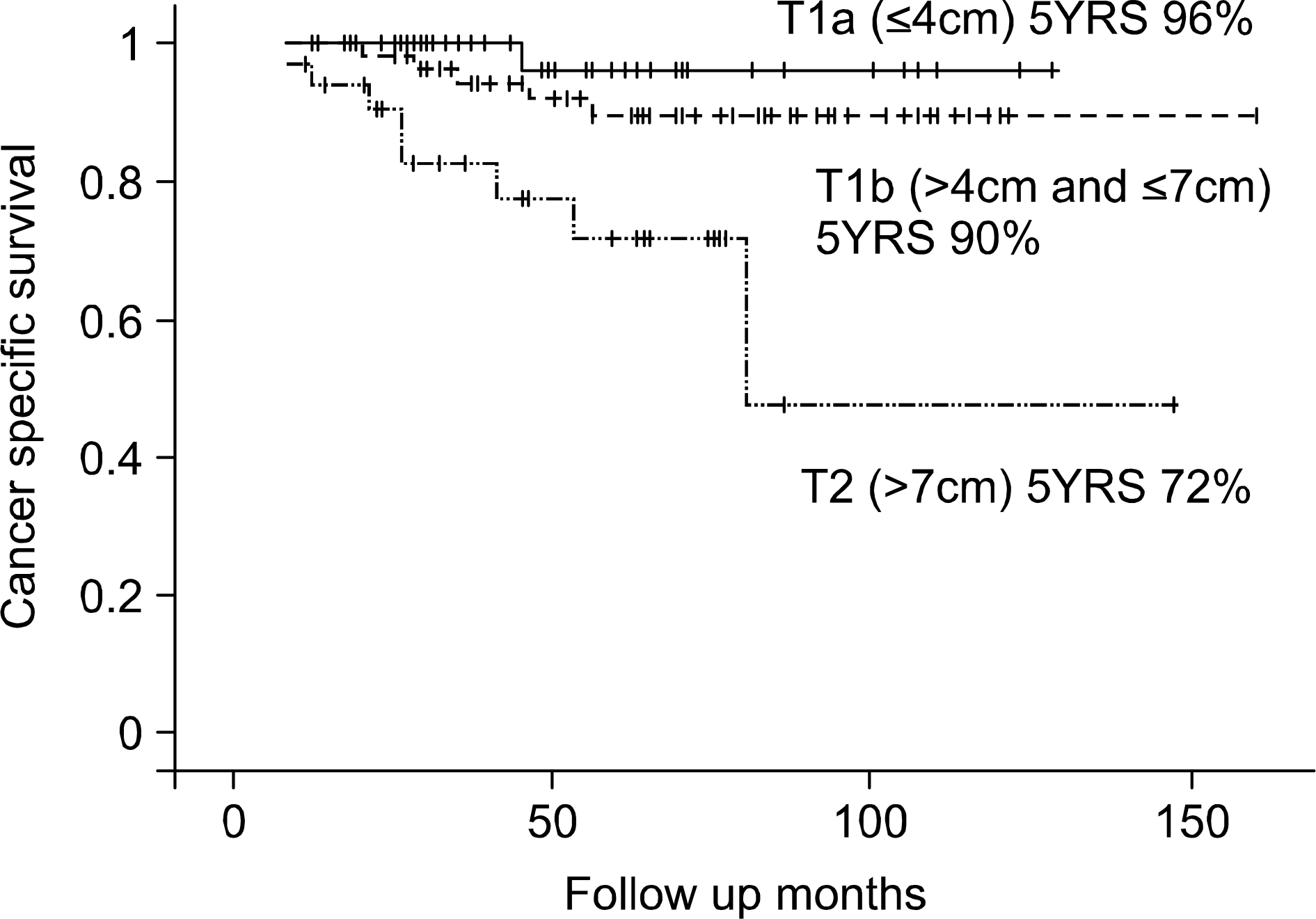
114 and 33 patients were pT1 (77.6%) and pT2 (22.4%), respectively. There were 10 recurrences (8.8%) and 6 deaths (5.3%) in the pT1 group, and 8 recurrences (24.2%) and 8 deaths (24.2%) in the pT2 group. The differences in survival were maximized when the tumor size cutoff point was 7cm (cancer-specific survival rate: 92.0% vs. 71.5% p=0.0003, diseasefree survival rate: 88.5% vs. 69.1% p=0.0092). The next significant difference was observed with a cutoff of 4cm (cancer-specific survival rate: 96.0% vs. 83.7% p=0.0467, disease-free survival rate: 96.0% vs. 78.8% p= 0.0121).
Conclusions:
Tumor size is an important prognostic factor in patients with an organ confined RCC. The established cutoff point of 7cm provided reasonable prognostic differences. A 4cm cutoff point is also feasible for separating groups with different survivals after a nephrectomy. Hence, the T1a/1b/T2 classification system is practical for the division of localized RCC. (Korean J Urol 2006;47:601-606)
Go to : 
REFERENCES
1.Baltaci S., Orhan D., Soyupek S., Beduk Y., Tulunay O., Gogus O. Influence of tumour stage, size, grade, vascular involvement, histological cell type and histological pattern on multi-focality of renal cell carcinoma. J Urol. 2000. 164:36–9.
2.Tsui K., Shvarts O., Smith RB., Figlin RA., De Kemion JD., Belldegrun A. Prognostic indicators for renal cell carcinoma: a multivariate analysis of 643 patients using the revised 1997 TNM staging criteria. J Urol. 2000. 163:1090–5.

3.Storkel S., Eble JN., Adlakha K., Amin M., Blute ML., Bostwick DG, et al. Classification of renal cell carcinoma: Workgroup No. 1. Union Internationale Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC). Cancer. 1997. 80:987–9.
4.Greene FL., Page DL., Fleming ID., Fritz A., Balch CM., Haller DG, et al. AJCC cancer staging manual. 6th ed.New York: Springer-Verlag;2002. ;323-8.
5.Thompson M., Peek M. Improvement in survival of patients with renal cell carcinoma– 仕Le role of the serendipitously detected tumor. J Urol. 1988. 140:487–90.
6.Bell ET. A classification of renal tumors with observation on the frequency of the various types. J Urol. 1938. 39:238–43.
7.Grignon DJ., Ayala AG., el-Naggar A., Wishnow KI., Ro JY., Swanson DA, et al. Renal cell carcinoma. A clinicopathologic and DNA flow cytometric analysis of 103 cases. Cancer. 1989. 64:2133–40.

8.Robson CJ., Churchill BM., Anderson W. The results of radical nephrectomy for renal cell carcinoma. J Urol. 1969. 101:297–301.

9.Javidan J., Strieker HJ., Tamboli P., Amin MB., Peabody JO., Deshpande A, et al. Prognostic significance of the 1997 TNM classification of renal cell carcinoma. J Urol. 1999. 162:1277–81.

10.Minervini R., Minervini A., Fontana C., Traversi C., Cristofani R. Evaluation of the 1997 tumour, nodes and metastases classification of renal cell carcinoma: experience in 172 patients. BJU Int. 2000. 86:199–202.

11.Wunderlich H., Dreihaupt M., Schlichter A., Kosmehl H., Rei-chelt O., Schubert J. New cut-off point between T1 and T2 renal cell carcinoma - necessary for a better discriminatory power of the TNM classification. Urol Int. 2004. 72:123–8.

12.Zisman A., Pantuck AJ., Chao D., Dorey F., Said JW., Gitlitz BJ, et al. Re-evaluation of the 1997 TNM classification for renal cell carcinoma: T1 and T2 cut-off point at 4.5 rather than 7cm. Better correlates with clinical outcome. J Urol. 2001. 166:54–8.
13.Elmore JM., Kadesky KT., Koeneman KS. Sagalowsky AL Reassessment of the 1997 TNM classification system for renal cell carcinoma. Cancer. 2003. 98:2329–34.
14.Gelb AB., Shibuya RB., Weiss LM., Medeiros LJ. Stage I renal cell carcinoma. A clinico-pathologic study of 82 cases. Am J Surg Pathol. 1993. 17:275–86.
15.Kinouchi T., Saiki S., Meguro N., Maeda O., Kuroda M., Usami M, et al. Impact of tumor size on the clinical outcomes of patients with Robson stage I renal cell carcinoma. Cancer. 1999. 85:689–95.

16.Ficarra V., Prayer-Galetti T., Novara G., Bratti E., Zanolla L., Dal Bianco M, et al. Tumor-size breakpoint for prognostic stratification of localized renal cell carcinoma. Urology. 2004. 63:235–9.

17.Hafez KS., Fergany AF., Novick AC. Nephron sparing surgery for localized renal cell carcinoma: impact of tumor size on patient survival, tumor recurrence and TNM staging. J Urol. 1999. 162:1930–3.

18.Matin SF., Gill IS., Worley S., Novick AC. Outcome of laparoscopic radical and open partial nephrectomy for the sporadic 4cm or less renal tumor with a normal contralateral kidney. J Urol. 2002. 168:1356–9.
19.Licht MR., Novick AC., Goormastic M. Nephron-sparing surgery in incidental versus suspected renal cell carcinoma. J Urol. 1994. 152:39–42.
20.Lemer SE., Hawkins CA., Blute ML., Grabner A., Wollan PC., Eickholt JT, et al. Disease outcome in patients with low stage renal cell carcinoma treated with nephron-sparing or radical surgery. J Urol. 1996. 155:1868–73.
21.Cheville JC., Blute ML., Zincke H., Lohse CM., Weaver AL. Stage pTl conventional (clear cell) renal cell carcinoma: pathologic features associated with cancer-specific survival. J Urol. 2001. 166:453–6.
22.Salama ME., Guru K., Strieker H., Peterson E., Peaboby J., Menon M, et al. pTl substaging in renal cell carcinoma: validation of the 2002 TNM staging modification of malignant renal epithelial tumors. J Urol. 2005. 173:1492–5.
23.Igarashi T., Tobe T., Nakatsu H., Suzuki N., Murakami S., Hamano M, et al. The impact of a 4-cm cut-o伴point for stratification of T1N0M0 renal cell carcinoma after radical nephrectomy. J Urol. 2001. 165:1103–6.
24.Kim DI., Park CH., Kim CI. Restaging of the stage T1 localized renal cell carcinomas using new cut-o伴value. Korean J Urol. 2005. 46:43–8.
25.Kim DS., Woo YN., Lee TY. The value of tumor size as a prognostic factor in patients with localized renal cell carcinomas. Korean J Urol. 2002. 43:813–7.
26.Lee SW., Kim YJ., Kim YW., Chang SG. The prognostic factors influencing the survival rate in patients with localized renal cell carcinoma. Korean J Urol. 2004. 45:872–7.
27.Lohse CM., Cheville JC. A review of prognostic pathologic features and algorithms for patients treated surgically for renal cell carcinoma. Clin Lab Med. 2005. 25:433–64.

28.Pantuck AJ., Zisman A., Belldegrun AS. The changing natural history of renal cell carcinoma. J Urol. 2001. 166:1611–23.

29.Lang H., Lindner V., Letoumeux H., Martin M., Saussine C., Jacqmin D. Progmostic value of microscopic venous invasion in renal cell carcinoma: long-term follow up. Eur Urol. 2004. 46:331–5.
Go to : 
Table 1.
Patient characteristics
Table 2.
Disease recurrence site in T1 and T2
| T1 | T2 | |||
|---|---|---|---|---|
| n | % | n | % | |
| Recurrence rate | 10/114 | 16.6 | 8/33 | 23.5 |
| Recurrence site | ||||
| Lung | 5 | 50.0 | 7 | 87.5 |
| Liver | 3 | 30.0 | 1 | 12.5 |
| Adrenal gland | 2 | 20.0 | 0 | 0.0 |
| Pleura | 1 | 10.0 | 0 | 0.0 |
| Bone | 1 | 10.0 | 1 | 12.5 |
| Brain | 0 | 0.0 | 1 | 12.5 |
Table 3.
Cancer specific survival and disease free survival according to tumor size
Table 4.
Univariate analysis of prognostic factors
Table 5.
Relationships between tumor size and other prognostic factors evaluated at 4 and 5cm cutoff points




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download