Abstract
Purpose
The purpose of this study was to compare the effects of resiniferatoxin (RTX) and botulinum toxin (BTX) on the bladder detrusor function in a cyclophosphamide (CYP)-induced cystitis rat model.
Materials and Methods
Sprague-Dawley rats were divided into 5 groups (1: saline treated, 2: CYP and BTX treated, 3: CYP and RTX treated, 4 and 5: CYP treated and sham operated as the counterpart of groups 2 and 3, respectively, with normal saline). 100mg/kg CYP was injected every third day for five weeks. Cystometrograms were performed after the BTX and RTX treatments.
Results
1. The normal control group and the CYP-treated only group. In the CYP-treated group, the time of micturition frequency, the maximal detrusor pressure on the cystometergram (Pvesmax at CMG), the maximal detrusor pressure on the pressure-flow study (Pvesmax at pr/flow) and the episodes of irregular contractions were increased. 2. The CYP-only group and the CYP/BTX or CYP/RTXtreated groups. In the CYP/BTX or CYP/RTX treated groups, the time of micturition frequency, the Pvesmax at CMG, the Pvesmax at pr/flow and the episode of irregular contractions were decreased. 3. The CYP/BTXtreated group and the CYP/RTXtreated group. There was no statistically significant difference between the two groups regarding micturition frequency, the PvesMax at CMG and the PvesMax at pr/flow, the Dhfo and the episodes of involuntary contractions (p>0.05).
Conclusions
Intravesical administration of BTX or RTX blocked the CYP-induced detrusor overactivity as was shown by the restoration of the micturition frequency, the intravesical pressure and the involuntary contraction episodes to a control level. There was no statistically significant difference between the two groups regarding the urodynamic parameters.
Figures and Tables
 | Fig. 1Triple lumen catheter, which is seated securely in the bladder neck for functional separation of the bladder and urethral activity. |
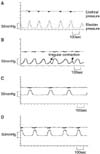 | Fig. 2Representative cystometric tracings of (A) the normal control group, (B) the cyclophosphamide (CYP)-injected group, (C) the CYP/botulinum toxin (BTX) treated group and (D) the CYP/resiniferatoxin (RTX) treated group. (A) The normal control group shows regular bladder contraction without any irregular bladder contraction. (B) The CYP-induced cystitis model shows concurrent irregular contractions (arrows) between the normal bladder contractions. (C) and (D) BTX and RTX injection, respectively, in the CYP-induced rat bladder shows recovery of regular bladder contractions without any involuntary bladder contractions. |
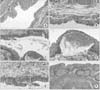 | Fig. 3Histological finding (H&E staining) of the normal and cyclophosphamide induces cystitis bladders after 2 weeks. (A) Histologic findings of the normal bladder, i.e., the epithelial cell lining (×100). (B) Histologic findings of the normal bladder, i.e., loose connective tissue (×200). (C) Interstitial edema and hemorrhage in the cyclophosphamide-induced cystitis bladder (×100). (D) Magnified findings of focal hemorrhage (×200). (E) Inflammatory cell infiltration in the cyclophosphamide-induced cystitis bladder (×200). (F) Wall thickening and lymphocyte infiltration in the cyclophosphamide induced cystitis bladder (×100). |
Table 1
Comparative urodynamic parameters between the normal control group and the cyclophosphamide treated group

References
1. Sant GR, LaRock DR. Standard intravesical therapies for interstitial cystitis. Urol Clin North Am. 1994. 21:73–83.
2. Birder LA, Kanai AJ, de Groat WC, Kiss S, Nealen ML, Burke NE, et al. Vanilloid receptor expression suggests a sensory role for urinary bladder epithelial cells. Proc Natl Acad Sci USA. 2001. 98:13396–13401.
3. Phelan MW, Franks M, Somogyi GT, Yokoyama T, Fraser MO, Lavelle JP, et al. Botulinum toxin urethral sphincter injection to restore bladder emptying in men and woman with voiding dysfunction. J Urol. 2001. 165:1107–1110.
4. Smith CP, Franks ME, McNeil BK, Ghosh R, de Groat WC, Chancellor MB, et al. Effect of botulinum toxin A on the autonomic nervous system of the rat lower urinary tract. J Urol. 2003. 169:1896–1900.
5. Avelino A, Cruz F, Coimbra A. Intravesical resiniferatoxin desensitizes rat bladder sensory fibers without causing intense noxious excitation: a c-fos study. Eur J Pharmacol. 1999. 378:17–22.
6. Lazzeri M, Beneforti P, Spinelli M, Zanollo A, Barbagi G, Turini D. Intravesical resiniferatoxin for the treatment of hypersensitivity disorder: a randomized placebo controlled study. J Urol. 2000. 164:676–679.
7. McMahon SB, Abel C. A model for the study of visceral pain states: chronic inflammation of the chronic decerebrate rat urinary bladder by irritant chemicals. Pain. 1987. 28:109–127.
8. Berkley KJ, Wood E, Scofield SL, Little M. Behavioral responses to uterine or vaginal distension in the rat. Pain. 1995. 61:121–131.
9. Lanteri-Minet M, Bon K, de Pommery J, Michiels JF, Menetrey D. Cyclophosphamide cystitis as a model of visceral pain in rats: model elaboration and spinal structures involved as revealed by the expression of c-Fos and Krox-24 proteins. Exp Brain Res. 1995. 105:220–232.
10. Fraiser LH, Kanekal S, Kehrer JP. Cyclophosphamide toxicity. Characterising and avoiding the problem. Drugs. 1991. 42:781–789.
11. de Groat WC. A neurologic basis for the overactive bladder. Urology. 1997. 50:36–52.
12. Bon K, Lichtensteiger CA, Wilson SG, Mogil JS. Characterization of cyclophosphamide cystitis, a model of viceral and referred pain, in the mouse: species and strain differences. J Urol. 2003. 170:1008–1012.
13. Vizzard MA. Up-regulation of pituitary adenylate cyclase-activating polypeptide in urinary bladder pathways after chronic cystitis. J Comp Neurol. 2000. 420:335–348.
14. Boucher M, Meen M, Codron JP, Coudore F, Kemeny JL, Eschalier A. Cyclophosphamide-induced cystitis in freely-moving conscious rats: behavioral approach to a new model of visceral pain. J Urol. 2000. 164:203–208.
15. Ferguson DR, Kennedy I, Burton TJ. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes: a possible sensory mechanism? J Physiol. 1997. 505:503–511.
16. Hohenfellner M, Black P, Linn JF, Dahms SE, Thuroff JW. Surgical treatment of interstitial cystitis in women. Int Urogynecol J Pelvic Floor Dysfunct. 2000. 11:113–119.
17. Zermann D, Ishigooka M, Schubert J, Schmidt RA. Perisphincteric injection of botulinum toxin type A. A treatment option for patients with chronic prostatic pain? Eur Urol. 2000. 38:393–399.
18. Schurch B, Stohrer M, Kramer G, Schmid DM, Gaul G, Hauri D. Botulinum-A toxin for treating detrusor hyperreflexia in spinal cord injured patients: a new alternative to anticholinergic drugs? Preliminary results. J Urol. 2000. 164:692–697.
19. Ruda MA, Iadarola MJ, Cohen LV, Young WS 3rd. In situ hybridization histochemistry and immunocytochemistry reveal an increase in spinal dynorphin biosynthesis in a rat model of peripheral inflammation and hyperalgesia. Proc Natl Acad Sci USA. 1988. 85:622–626.
20. Anderson KE. Pharmacology of lower urinary tract smooth muscles and penile erectile tissues. Pharmacol Rev. 1993. 45:253–308.
21. Magnan A, Bettelini L, Hagan RM, Pietra C. Effects of intrathecal NK-1 and NK-2 antagonists on xylene-induced cystitis in rats. Neuropeptides. 1993. 24:199–200.
22. Ishigooka M, Zermann DH, Doggweiler R, Schmit RA, Hashimoto T, Nakada T. Spinal NK1 receptor is upregulated after chronic bladder irritation. Pain. 2001. 93:43–50.
23. Dinis P, Charrua A, Arelino A, Cruz F. Intravesical resiniferatoxin decreases spinal c-fos expression and increases bladder volume to reflex micturition in rats with chronic inflamed urinary bladders. BJU Int. 2004. 94:153–157.
24. Silva C, Rio ME, Cruz F. Desensitization of bladder sensory fibers by intravesical resiniferatoxin, a capsaicin analog: long-term results for the treatment of detrusor hyperreflexia. Eur Urol. 2000. 38:444–452.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download