Abstract
Purpose
The process of transferring data from one medical center to another and sharing the results can be time-consuming and inconvenient. So, we introduce the multi-institutional clinical research that uses the Internet for conducting faster, safer and more convenient research.
Materials and Methods
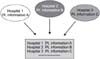
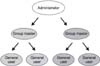
Patients with benign prostatic hyperplasia (BPH) were prospectively enrolled from 3 remote participating medical centers by means of the Internet. When a patient with BPH came to the hospital, the BPH profile, medications and adverse events were entered into the Internet Based Multi-institute Prostate Research System (IBMPRS). Six investigators were educated on the use of the IBMPRS and we measured the mean data entering time and the user satisfaction.
Results
763 patients with BPH from three institutions were enrolled and their data was collected through the Internet for 1 year. It took an average of 2 minutes 43 seconds to enter the information of one patient. All the investigators were satisfied about the IBMPRS and the system was very stable and safe.
Conclusions
An Internet-based clinical research system has the advantage of being able to instantly share patient information and full-time access to the interim results is available if a large number of institutions participate in the clinical research. Clinical research using the Internet would be an invaluable method that is free to use with easy accessibility, and it could play the role of an excellent secretary on the web.
Figures and Tables
References
1. Chung BH, Yang KM, Hong SJ. Meta-analysis of alpha receptor antagonist for benign prostatic hyperplasia from papers that were published in Korea. Korean J Urol. 2005. 46:252–258.
2. Lallas CD, Preminger GM, Pearle MS, Leveillee RJ, Lingeman JE, Schwope JP, et al. Internet based multi-institutional clinical research: a convenient and secure option. J Urol. 2004. 171:1880–1885.
3. Lindh JD, Kublickas M, Westgren M, Rane A. Internet based clinical trial protocols as applied to a study of warfarin pharmacogenetics. Br J Clin Pharmacol. 2004. 58:482–487.
4. Lopez-Carrero C, Arriaza E, Bolanos E, Ciudad A, Municio M, Ramos J, et al. Internet in clinical research based on a pilot experience. Contemp Clin Trials. 2005. 26:234–243.
5. Avidan A, Weissman C, Sprung CL. An internet web site as a data collection platform for multicenter research. Anesth Analg. 2005. 100:506–511.
6. Food and drug administration. Title 21 code of federal regulations (21 CFR part 11). "electronic records; electronic signatures.".




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download