Calcifying fibrous pseudotumor (CFP) is an unusual benign lesion of the soft tissue. CFP is regarded as related to inflammatory pseudotumor and it may sometimes represent a late stage of inflammatory pseudotumor, in that a CFP appears to be arising within an inflammatory pseudotumor.1 This entity occurs predominantly in young adult patients and has a good prognosis.2 CFPs are found mostly in the extremities, neck, trunk, pleura, and mediastinum, but rarely in the spermatic cord.3-5
We report a case of CFP of the spermatic cord in a 24-year-old man cured by a simple excision.
CASE REPORT
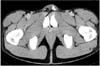
A 24-year-old man presented with a palpable painless left inguinal mass which had been present for 3 months before visiting our outpatient clinic. He had no history of any systemic disease. Physical examination revealed a 2cm sized non tender firm mass in the left inguinal area. Laboratory analyses of blood and urine samples were unremarkable. Abdominal computed tomography scan disclosed a 1.6cm sized soft tissue mass with amorphous calcification along the left spermatic cord, prompting to the diagnosis of a calcified granuloma (Fig. 1).
The surgical excision was performed through a left inguinal incision. The mass was located along the left spermatic cord. During the dissection of the mass from the spermatic cord, there was no adhesion.
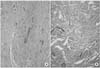
On gross examination, the mass measured at 2.0×1.0×1.0cm in dimension. It was well circumscribed, lobulated and solid, but non-encapsulated. On section, the cut surface showed a gritty texture and a varied appearance with irregular calcifications. Microscopically, there were areas of diffuse and extensive calcifications with numerous psammomatous calcifications and other areas were densely collagenized and in focal areas of the mass, there were patchy infiltrates of plasma cells and lymphocytes (Fig. 2). The gross and microscopic findings were compatible with CFP of the spermatic cord.
The patient was discharged on the first postoperative day. He has been asymptomatic and there was no evidence of recurrence at 7 months postoperatively.
DISCUSSION
CFP is a rare benign fibrous lesion that was first reported by Rosenthal and Abdul-Karim in 1988. It was previously termed childhood fibrous tumor with psammoma bodies.6 In 1993, Fetsch et al.7 described the lesion in a series of 10 patients, and termed it calcifying fibrous pseudotumor. CFP occurs predominantly in the soft tissues of young adults with some predisposition for female. The patients involved are mainly in their first to third decade of life. It is discovered as a slow-growing painless palpable soft tissue mass in the extremities, neck, trunk, pleura, peritoneum, and mediastinum. CFP ranges in size from 25 to 150mm, with larger lesions tending to occur in the soft tissues of the extremities and smaller lesions in the subserosal sites. Two cases of CFP of the spermatic cord were reported previously in the literature. Nascimento et al.3 reported a 4 cm sized CFP of the spermatic cord in a 21-year-old male and Zamecnik et al.4 reported a similar case of a 2 cm sized CFP of the spermatic cord in a 19-year-old male. Our case was similar in age at onset and clinical features to previously reported cases.
The cause and pathogenesis of CFP is uncertain. Some suggest that CFP represents the late stage of an inflammatory myofibroblastic tumor, while others propose that CFP may result from a reactive inflammatory process.8,9 The diagnosis of CFP can be confirmed histopathologically with the hematoxylineosin stain. CFP is characterized by hyalinized collagen with psammomatous or dystrophic calcifications, lymphocytes and some plasma cells.10 Mitotic activity, cellular atypia, tissue invasion or desmoplasia is absent in CFP. The differential diagnoses of CFP include the inflammatory myofibroblastic tumor, calcified granuloma, solitary fibrous tumor and amyloidoma. In our case, all the typical findings of a CFP were observed using the hematoxylin-eosin stain, allowing the prompt diagnostic confirmation as a CFP.
Usually, a CFP has an excellent prognosis. It rarely recurs locally, but if it does, a simple re-excision is curative. Systemic metastasis was not reported.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download