Abstract
Purpose
The neuroendocrine cell (NE cell) is thought to play an important role in the development of hormone-refractory prostate cancer. Survivin is one of the IAPs (inhibitors of apoptosis), and it is expressed in the NE cell and in most of the common cancers, but not in normal tissue. The objective of this study was to investigate the expression pattern of the NE cell and survivin in the prostate of rabbits.
Materials and Methods
The 9 rabbits underwent orchiectomy and their prostates were removed at 0 weeks (control), 2 weeks and 6 weeks after orchiectomy. Each of the prostatic tissue specimens was stained with H&E; immunohistochemical staining was done for chromogranin A, synaptophysin and survivin, and the tissue specimens were then examined by microscopy.
Results
In the prostate of rabbits, most of the NE cells were located between the epithelial gland and the stroma. NE differentiation occurred 6 weeks after orchiectomy. The location of cells that were positive for survivin was almost same as that of the NE cells.
Conclusions
The main location of NE cells in the prostate of rabbits was between the epithelial gland and the stroma, and NE differentiation occurred 6 weeks after orchiectomy, the same as in a human or a dog. The location of survivin positive cells coincided with that of the NE cells. Therefore, a rabbit seems to be a suitable animal model for the study of the NE cell.
Figures and Tables
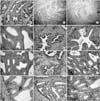 | Fig. 1Hematoxylin Eosin stain (A-C) and immunohistochemical stain with monoclonal antibodies to CgA (D-F), SNP (G-I) and survivin (J-L) in the prostate of rabbits before orchiectomy (A, D, G, J), 2 weeks after (B, E, H, K), and 6 weeks after orchiectomy (C, F, I, L) (×100: A-C) (×400: D-L). In the prostate of rabbits, the shape of the acini became shrunken and the number of epithelial cells are decreased after orchiectomy. Although most NE cells are located between the stroma and the glandular epithelium, there is the small number of NE cells in the glandular epithelium, as noted on the SNP staining. NE differentiation is found 6 weeks after of orchectomy. Also, the cells that have a positive reaction to survivin are found in all of the three groups. The location of the cells positive to survivin is compatible with that of the NE cell, and the number of cells is increased after 4 weeks. |
Table 1
Gross and microscopic findings of the rabbit prostate

0: 0-5% in number of positive cells, +: 2-25% in number of positive cells, ++: more than 25% in number of positive cells, 0+: no or low intensity of stain, 1+: moderate to strong intensity of stain, NE cell: neuroendocrine cell, pathologic finding in H-E stain: per low power field (×100), pathologic finding in immunohistochemical stain: (×400)
References
1. Aprikian AG, Cordon-Cardo C, Fair WR, Reuter VE. Charac terization of neuroendocrine differentiation in human benign prostate and prostatic adenocarcinoma. Cancer. 1993. 71:3952–3965.
2. Abrahamsson PA. Neuroendocrine differentiation in prostatic carcinoma. Prostate. 1999. 39:135–148.
3. Angelsen A, Mecsei R, Sandvik AK, Waldum HL. Neuroendocrine cells in the prostate of the rat, guinea pig, cat, and dog. Prostate. 1997. 33:18–25.
4. Xing N, Qian J, Bostwick D, Bergstralh E, Young CY. Neuroendocrine cells in human prostate over-express the anti-apoptosis protein survivin. Prostate. 2001. 48:7–15.
5. Helpap B. Morphology and therapeutic strategies for neuroendocrine tumors of the genitourinary tract. Cancer. 2002. 95:1415–1420.
6. Xue Y, Verhofstad A, Lange W, Smedts F, Debruyne F, de la Rosette J, et al. Prostatic neuroendocrine cells have a unique keratin expression pattern and do not express Bcl-2: cell kinetic features of neuroendocrine cells in the human prostate. Am J Pathol. 1997. 151:1759–1765.
7. Bonkhoff H. Neuroendocrine cells in benign and malignant prostate tissue: morphogenesis, proliferation, and androgen receptor status. Prostate. 1998. 8:Suppl. 18–22.
8. Bonkhoff H, Stein U, Remberger K. Endocrine-paracrine cell types in the prostate and prostatic adenocarcinoma are postmitotic cells. Hum Pathol. 1995. 26:167–170.
9. Cohen RJ, Glezerson G, Taylor LF, Grundle HA, Naude JH. The neuroendocrine cell population of the human prostate gland. J Urol. 1993. 150:365–368.
10. Ismail A HR, Landry F, Aprikian AG, Chevalier S. Androgen ablation promotes neuroendocrine cell differentiation in dog and human prostate. Prostate. 2002. 51:117–125.
11. di Sant'Agnese PA. Neuroendocrine differentiation in human prostatic carcinoma. Hum Pathol. 1992. 23:287–296.
12. Aprikian AG, Cordon-Cardo C, Fair WR, Reuter VE. Characterization of neuroendocrine differentiation in human benign prostate and prostatic adenocarcinoma. Cancer. 1993. 71:3952–3965.
13. Yamamoto T, Tanigawa N. The role of survivin as a new target of diagnosis and treatment in human cancer. Med Electron Microsc. 2001. 34:207–212.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download