Abstract
Purpose
To explore the expressions of p53 and phosphorylation-H2AX in varicocele-induced rat testes.
Materials and Methods
A total of 16 adult male Sprague-Dawley rats underwent an operation; 12 underwent an experimental varicocele and 4, as controls, were sham-operated. Groups of 4 varicocele-induced rats underwent a left orchiectomy after 2 or 3 weeks, or both orchiectomies after 4 weeks. The sham-operated rats underwent both orchiectomies after 4 weeks. Sections of both testes from each animal were studied. The changes in the expressions of p53 and phosphorylation of H2AX were determined using immunohistochemistry and western blot.
Results
Immunohistochemical staining of the left testes in the varicocele-induced rats showed that the expressions of p53 and phosphorylation of H2AX had not begun 2 weeks postoperatively, but remarkable results were observed after 3 and 4 weeks. Both testes of the varicocele-induced rats showed the expressions of p53 and phosphorylation of H2AX after 4 weeks, with the left testes being more distinctive in immunohistochemical staining compared to the right. Western blot of the left testes in the varicocele-induced rats also showed unclear expressions of p53 and γ-H2AX after 2 weeks. Considerable distinction was seen after 3 and 4 weeks compared to the control group.
Figures and Tables
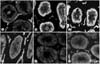 | Fig. 1Immunohistochemical staining for p53 in varicocele-induced rats. Expressions of p53 at 3 and 4 weeks (B, C and D) are increased compared to the control group (E and F). (A) Left testis of the varicocele-induced rat after 2 weeks, (B) Left testis of the varicocele-induced rats after 3 weeks, (C) Left testis of the varicocele-induced rats after 4 weeks, (D) Right testis of the varicocele-induced rats after 4 weeks, (E) Left testis of the sham-operated rats after 4 weeks (control group), (F) Right testis of the sham-operated rats after 4 weeks (control group). |
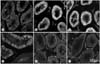 | Fig. 2Immunohistochemical staining for γ-H2AX in the varicocele rats. Expressions of phosphorylation of H2AX after 3 and 4 weeks (B, C and D) are increased compared to control group (E and F). (A) Left testis of the varicocele-induced rat after 2 weeks, (B) Left testis of the varicocele-induced rats after 3 weeks, (C) Left testis of the varicocele-induced rats after 4 weeks, (D) Right testis of the varicocele-induced rats after 4 weeks, (E) Left testis of the sham-operated rats after 4 weeks (control group), (F) Right testis of the sham-operated rats after 4 weeks (control group). |
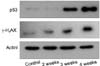 | Fig. 3Western blotting of the p53 and γ-H2AX expressed in the varicocele-induced rat testes. Protein extracted from the rat testis, with (2, 3 and 4 weeks) or without (4 weeks) partial ligation of the Lt renal vein, is analyzed for the expression levels of p53, γ-H2AX and actin by Western blotting. The expressions of p53 and γ-H2AX after weeks 3 and 4 are significantly higher compared to control group and the varicocele-induced rats 2 weeks postoperatively. |
References
1. Naughton CK, Nangia AK, Agarwal A. Pathophysiology of varicoceles in male infertility. Hum Reprod Update. 2001. 7:473–481.
2. Fujisawa M, Hiramine C, Tanaka H, Okada H, Arakawa S, Kamidono S. Decrease in apoptosis of germ cells in the testes of infertile men with varicocele. World J Urol. 1999. 17:296–300.
3. Agarwal A, Allamaneni SS. Sperm DNA damage assessment: a test whose time has come. Fertil Steril. 2005. 84:850–853.
4. Ku J, Shim HB, Kim SW, Paick JS. The role of apoptosis in the pathogenesis of varicocele. BJU Int. 2005. 96:1092–1096.
5. Saleh RA, Agarwal A, Sharma RK, Said TM, Sikka SC, Thomas AJ. Evaluation of nuclear DNA damage in spermatozoa from infertile men with varicocele. Fertil Steril. 2003. 80:1431–1436.
6. Agarwal A, Said TM. Oxidative stress, DNA damage and apoptosis in male infertility: a clinical approach. BJU Int. 2005. 95:503–507.
7. Sinha Hikim AP, Swerdloff RS. Hormonal and genetic control of germ cell apoptosis in the testis. Rev Reprod. 1999. 4:38–47.
8. Huckins C. The morphology and kinetics of spermatogonial degeneration in normal adult rats: an analysis using a simplified classification of the germinal epithelium. Anat Rec. 1978. 190:905–926.
9. Fisher DE. The p53 tumor suppressor: critical regulator of life and death in cancer. Apoptosis. 2001. 6:7–15.
10. Lugar K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature. 1997. 389:251–260.
11. Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phophorylation on serine 139. J Biol Chem. 1998. 273:5858–5868.
12. Rogakou EP, Boon C, Redon C, Bonner WM. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol. 1999. 146:905–916.
13. Sedelnikova OA, Rogakou EP, Panyutin IG, Bonner WM. Quantitative detection of (125)IdU-induced DNA double-strand breaks with gamma-H2AX antibody. Radiat Res. 2002. 158:486–492.
14. Gorelick JI, Goldstein M. Loss of infertility in men with varicocele. Fertil Steril. 1993. 59:613–616.
15. Greenberg SM, Lipschultz LI, Wein AJ. Experience with 425 subfertile male patients. J Urol. 1978. 119:507–512.
16. Sayfan J, Halevy A, Oland J, Nathan H. Varicocele and left renal vein compression. Fertil Steril. 1984. 41:411–417.
17. Schneck FX, Bellinger MF. Walsh PC, Retik AB, Vaughan ED, Wein AJ, editors. Abnormalities of the testis and scrotum and their surgical management. Campbell's urology. 1998. 8th ed. Philadelphia: Saunders;2384–2388.
18. Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implication in tissue kinetics. Br J Cancer. 1972. 26:239–257.
19. Barqawi A, Caruso A, Meacham RB. Experimental varicocele induces testicular germ cell apoptosis in the rat. J Urol. 2004. 171:501–503.
20. Simsek F, Turkeri L, Cevik I, Bircan K, Akdas A. Role of apoptosis in testicular tissue damage caused by varicocele. Arch Esp Urol. 1998. 51:947–950.
21. Kilinc F, Guvel S, Kayaselcuk F, Aygun C, Egilmez T, Ozkardes H. p53 expression and apoptosis in varicocele in the rat testis. J Urol. 2004. 172:2475–2478.
22. Tanaka H, Fujisava M, Tanaka H, Okada H, Kamidono S. Apoptosis-related proteins in the testes of infertile men with varicocele. BJU Int. 2002. 89:905–909.
23. Hendin BN, Kolettis PN, Sharma RK, Thomas AJ, Agarwal A. Varicocele is associated with elevated speratozoal reactive oxygen species production and diminished seminal plasma antioxidant capacity. J Urol. 1999. 161:1831–1834.
24. Lane DP. Cancer. p53, guardian of the genome. Nature. 1992. 358:15–16.
25. Henriksen K, Kangasniemi M, Parvinen M, Kaipia A, Hakovirta H. In vitro, follicle-stimulating hormone prevents apoptosis and stimulates deoxyribonucleic acid synthesis in the rat seminiferous epithelium in a stage-specific fashion. Endocrinology. 1996. 137:2141–2149.
26. Khanna KK, Jackson SP. DNA double-strand breaks: signaling, repair and the cancer connection. Nat Genet. 2001. 27:247–254.
27. Zweidler A. Complexity and variability of the histone complement. Life Sci Res. 1976. 4:187–196.
28. Wu RS, Panusz HT, Hatch CL, Bonner WM. Histones and their modifications, CRC crit. Rev Biochem. 1986. 20:201–263.
29. Chen HT, Bhandoola A, Difilippantonio MJ, Zhu J, Brown MJ, Tai X, et al. Response to RAG-mediated VDJ cleavage by NBS1 and gamma-H2AX. Science. 2000. 290:1962–1965.
30. Goldberg M, Stucki M, Falck J, D'Amours D, Rahman D, Pappin D, et al. MDC1 is required for the intra-S-phase DNA damage checkpoint. Nature. 2003. 421:952–956.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download