Abstract
Purpose
Benign prostatic hyperplasia (BPH) is one of the common diseases in elderly men. Recently, the old-aged population has increased, with the interest in the clinical importance of BPH ever growing. Catechin, an extract of green tea, has the effect of the 5-alpha reductase inhibitor. Typically, BPH has been shown to be influenced by 5-alpha reductase. Therefore, the relationship between BPH and catechin was evaluated.
Materials and Methods
An experimental prostatic hyperplasia was induced in male Wistar rats by the administration of testosterone propionate, 3mg/kg sc, for 4 weeks. The Wistar rats were divided into four experimental groups: the control, BPH-induced, oral finasteride ingestion and oral catechin ingestion groups. After 4 weeks, the prostates were removed, and analyzed for their prostatic weight and histological examination.
Results
The prostate weights were measured in each group, and found to be 330.0±40.7, 970.0±1.1, 358.0±39.9 and 415.0±45.3mg in the control, BPH-induced, oral finasteride ingestion and oral catechin ingestion groups, respectively. The oral finasteride and catechin ingestion groups showed statistically significant decreases in their prostatic weights compared with the BPH-induced group (p<0.05), but with no significant difference between the oral finasteride and catechin ingestion groups (p>0.05). Histologically injected testosterone lead to prostatic hyperplasia in rats, but oral catechin ingestion decreased this change.
Figures and Tables
 | Fig. 1The mean weight of the specimen of the prostate in the rat model of benign prostatic hyperplasia, 4 weeks after the testosterone injection and in control group. (A) Control group, (B) testosterone injection group, (C) oral finasteride group, (D) oral catechin group. *: significantly different from B group (p<0.05), †: significantly different from B group (p<0.05). |
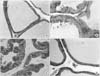 | Fig. 2Histologic findings from specimen of the prostate in the rat model of benign prostatic hyperplasia, 4 weeks after the testosterone injection and in the control group. (A) Secretory luminal cells, lined with a single layer of low columnar epithelium, and the acinus filled with pale eosinophilic material in the control rat (H&E, ×200). (B) Epithelial cell hyperplasia and fibrovascular stromal thickening (prostatic hyperplasia) in the testosterone injected rat (H&E, ×400). (C) Restricted proliferation of epithelial cells and the absence of stromal connective tissue proliferation in the finasteride treated rat (H&E, ×400). (D) Restricted proliferation of epithelial cells and the absence of stromal connective tissue proliferation in the catechin treated rat. Note the normal low columnar epithelium (H&E, ×200). |
References
1. McConnell JD. Prostate growth: new insights into hormonal regulation. Br J Urol. 1995. 76:Suppl 1. 5–10.
2. Coffey DS, Walsh PC. Clinical and experimental studies of benign prostatic hyperplasia. Urol Clin North Am. 1990. 17:461–475.
3. Wasson JH, Reda DJ, Bruskewitz RC, Elinson J, Keller AM, Henderson WG. A comparison of transurethral surgery with watchful waiting for moderate symptoms of benign prostatic hyperplasia. The Veterans Affairs Cooperative Study Group on transurethral resection of the prostate. N Engl J Med. 1995. 332:75–79.
4. Mebust WK, Holtgrewe HL, Cockett AT, Peters PC. Transurethral prostatectomy: immediate and postoperative complications. Cooperative study of 13 participating institutions evaluating 3,885 patients. J Urol. 2002. 167:5–9.
5. Gormley GJ. Evaluation of men on finasteride. Semin Urol Oncol. 1996. 14:139–144.
6. Boyle P, Gould AL, Roehrborn CG. Prostate volume predicts outcome of treatment of benign prostatic hyperplasia with finasteride: meta-analysis of randomizes clinical trials. Urology. 1996. 48:398–405.
7. Liao S, Kao YH, Hiipakka RA. Green tea: biochemical and biological basis for health benefits. Vitam Horm. 2001. 62:1–94.
8. Nagai K, Jiang MH, Hada J, Nagata T, Yajima Y, Yamamoto S, et al. (-)-Epigallocatechin gallate protects against NO stress-induced neuronal damage after ischemia by acting as an anti-oxidant. Brain Res. 2002. 956:319–322.
9. Morre DJ, Morre DM, Sun H, Cooper R, Chang J, Janle EM. Tea catechin synergies in inhibition of cancer cell proliferation and of a cancer specific cell surface oxidase (ECTO-NOX). Pharmacol Toxicol. 2003. 92:234–241.
10. Liao S. The medicinal action of androgens and green tea epigallocatechin gallate. Hong Kong Med J. 2001. 7:369–374.
11. Stanford NL, Searie JW, Kerr JF. Successive waves of apoptosis in the rat prostate after repeated withdrawal of testosterone stimulation. Pathology. 1984. 16:406–410.
12. Maggi CA, Manzini S, Giuliani S, Meli A. Infravesical outflow obstruction in rats: a comparison of two models. Gen Pharmacol. 1989. 20:345–349.
13. Welch G, Weinger K, Barry MJ. Quality-of-life impact of lower urinary tract symptom severity: results from the health professionals follow-up study. Urology. 2002. 59:245–250.
14. Jacobson SJ, Jacobson DJ, Griman CJ, Roberts RO, Rhodes T, Guess HA, et al. Natural history of rostatism: risk factors for acute urinary retention. J Urol. 1997. 158:481–487.
15. Barry MJ, Fowler FJ Jr, Bin L, Pitts JC III, Harris CJ, Mulley AG Jr. The natural history of patients with benign prostatic hyperplasia as diagnosed by North American urologists. J Urol. 1997. 157:10–14.
16. Population projections for Korea. 2001. Korea National Statistical Office.
17. 2001 Life Tables for Korea. 2001. Korea National Statistical Office.
18. Mcconnel JD. The pathophysiology of benign prostatic hyperplasia. J Androl. 1991. 12:356–363.
19. Boyle P, Maisonneuve P, Steg A. Decrease in mortality from benign prostatic hyperplasia: a major unheralded health triumph. J Urol. 1996. 155:176–180.
20. Walsh PC, Hutchins GH, Ewing LL. Tissue content of dihydrotestosterone in human prostatic hyperplasia is not supranormal. J Clin Invest. 1983. 72:1772–1777.
21. Birkhoff JD, Wiederhorn AR, Hamilton ML, Zinsser HH. Natural history of benign prostatic hypertrophy and acute urinary retention. Urology. 1976. 7:48–52.
22. Abrams P. Objective evaluation of bladder outlet obstruction. Br J Urol. 1995. 76:Suppl 1. 11–15.
23. Wilkin RP, Bruchovsky N, Rennie PS, Comeau TL. Stromal localization of testosterone 5α-reductase in normal, hyperplastic and carcinomatous prostates. Proc Am Assoc Cancer. 1979. 20:419.
24. Curtis CL, Harwood JL, Dent CM, Caterson B. Biological basis for the benefit of nutraceutical supplementation in arthritis. Drug Discov Today. 2004. 9:165–172.
25. Chung JH, Han JH, Hwang EJ, Seo JY, Cho KH, Kim KH, et al. Dual mechanisms of green tea extract (EGCG)-induced cell survival in human epidermal keratinocytes. FASEB J. 2003. 17:1913–1915.
26. McKay DL, Blumberg JB. The role of tea in human health: an update. J Am Coll Nutr. 2002. 21:1–13.
27. Negishi H, Xu JW, Ikeda K, Njelekela M, Nara Y, Tamori Y. Black and green tea polyphenols attenuate blood pressure inreases in stroke-prone spontaneously hypertensive rats. J Nutr. 2004. 134:38–42.
28. Liao S, Lin J, Dang MT, Zhang H, Kao YH, Fukuchi J, et al. Growth suppression of hamster flank organs by topical application of catechin, alizarin, curcumin, and myristoleic acid. Arch Dermatol Res. 2001. 293:200–205.
29. Mitra SK, Sundaram R, Mohan AR, Gopumadhavan S, Venkataranganna MV, Venkatesha U, et al. Protective effect of Prostane in experimental prostatic hyperplasia in rats. Asian J Androl. 1999. 1:175–179.
30. Liao S, Hiipakka RA. Selective inhibition of steroid 5α-reductase isozymes by tea epicatechin-3gallate and epigallocatechin-3-gallate. Biochem Biophys Res Commun. 1995. 214:833–838.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download