Abstract
Purpose
The objective of this study was to evaluate the role of microsatellite instability (MSI) in upper tract transitional cell carcinomas (TCC).
Materials and Methods
A total of 48 surgically treated renal pelvis and ureteral TCC patients were included in this study. MSI was determined by polymerase chain reaction (PCR) for amplification of the microsatellite sequences at mononucleotides BAT25 and BAT26, and dinucleotides D17S250, D2S123 and D5S346 in DNA and hMLH1 protein, p53 and Ki-67 expressions were determined by immunohistochemistry on retrieved tumor tissue.
Results
Twenty seven (56.2%) and 21 (43.8%) of the 48 patients had renal pelvis and ureteral TCC, respectively. Eighteen (37.5%) and 30 (62.5%) patients had superficial and invasive TCC, according to the pathological stage, while 24 each (50%) had low and high grade TCC, respectively. MSI was observed in 20.8% of tumors at mono-MSI and 22.9% at di-MSI. MSI-high and -low (instability >2 and <2 loci, respectively) were observed in 12.5 and 22.9%, respectively. From a univariate analysis, age, stage, tumor grade, tumor recurrence and the expressions of hMLH1 and Ki-67 were not related to MSI. However, in recurred cases, infiltrative tumors had 100% MSI compared to superficial tumors, and the expressions of p53 and Ki-67 were related to the stage and tumor grade, respectively (p<0.05).
Conclusions
These results suggest that MSI may occur in upper tract TCC, as observed in other tumors. MSI was more frequently expressed in ureteral than renal pelvis tumors. However, MSI was not correlated with stage or grade, but was significantly correlated with the stage in recurred cases; and the expressions of p53 and Ki-67 were related to the stage and tumor grade.
Figures and Tables
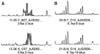 | Fig. 1Microsatellite analysis between normal DNA (upper) and tumor DNA (lower). (A) A case that is microsatellite unstable. In the tumor DNA, an additional band can be observed. (B) A case that is microsatellite stable. No MSI is observed in this case. MSI: microsatellite instability. |
References
1. Bishop JM. Molecular themes in oncogenesis. Cell. 1991. 64:235–248.
2. Sidransky D, Messing E. Molecular genetics and biochemical mechanisms in bladder cancer. Oncogenes, tumor suppressor genes, and growth factors. Urol Clin North Am. 1992. 19:629–639.
3. Loeb LA. Mutator phenotype may be required for multistage carcinogenesis. Cancer Res. 1991. 51:3075–3079.
4. Chu EH, Boehnke M, Hanash SM, Kuick RD, Lamb BJ, Neel JV, et al. Estimation of mutation rates based on the analysis of polypeptide constituents of cultured human lymphoblastoid cells. Genetics. 1988. 119:693–703.
5. Loeb LA. Microsatellite instability: marker of a mutator phenotype in cancer. Cancer Res. 1994. 54:5059–5063.
6. Peltomaki P, Lothe RA, Aaltonen LA, Pylkkanen L, Nystrom-Lahti M, Seruca R, et al. Microsatellite instability is associated with tumors that characterize the hereditary non-polyposis colorectal carcinoma syndrome. Cancer Res. 1993. 53:5853–5855.
7. Matsumura Y, Tarin D. DNA fingerprinting survey of various human tumors and their metastases. Cancer Res. 1992. 52:2174–2179.
8. Hall MC, Womack S, Sagalowsky AI, Carmody T, Erickstad MD, Roehrborn CG. Prognostic factors, recurrence, and survival in transitional cell carcinoma of the upper urinary tract: a 30-year experience in 252 patients. Urology. 1998. 52:594–601.
9. Messing EM. Walsh PC, Retik AB, Vaughan ED, Wein AJ, editors. Urothelial tumors of the urinary tract. Campbell's urology. 2002. 8th ed. Philadelphia: Saunders;2732–2784.
10. Tawfiek ER, Bagley DH. Upper-tract transitional cell carcinoma. Urology. 1997. 50:321–329.
11. Shinohara M, Okazawa A, Suzuki M, Itakura H, Munakata A, Kinoshita K. Clinical investigation of renal pelvis and ureteral cancer with special reference to adjuvant chemotherapy. Nippon Hinyokika Gakkai Zasshi. 1995. 86:1375–1382.
12. Hall MC, Womack S, Sagalowsky AI, Carmody T, Erickstad MD, Roehrborn CG. Prognostic factors, recurrence, and survival in transitional cell carcinoma of the upper urinary tract: a 30-year experience in 252 patients. Urology. 1998. 52:594–601.
13. Ozsahin M, Zouhair A, Villa S, Storme G, Chauvet B, Taussky D, et al. Prognostic factors in urothelial renal pelvis and ureter tumours: a multicentre rare cancer network study. Eur J Cancer. 1999. 35:738–743.
14. Sobin LH, Witterkind C. TNM classification of malignant tumors. 1997. 1st ed. New York: Wiley-Liss;183–186.
15. Park S, Hong B, Kim Y, Kim CS, Ahn H. Prognostic factors for survival in the transitional cell carcinoma of the upper urinary tract. Korean J Urol. 2003. 44:1087–1092.
16. Jeong IG, Kwak C, Jeong H, Lee ES, Lee CW, Lee SE. Carcinoma of the upper urinary tract: clinical analysis on patients during recent 10 years. Korean J Urol. 2003. 44:22–27.
17. Kim KH, Park JS, Kim CI, Lee KS. Risk factors for the development of bladder transitional cell carcinoma following surgery for transitional cell carcinoma of the upper urinary tract. Korean J Urol. 2005. 46:229–233.
18. Miyake H, Hara I, Arakawa S, Kamidono S. A clinicopathological study of bladder cancer associated with upper urinary tract cancer. BJU Int. 2000. 85:37–41.
19. Roupret M, Azzouzi AR, Cussenot O. Microsatellite instability and transitional cell carcinoma of the upper urinary tract. BJU Int. 2005. 96:489–492.
20. Vaish M, Mandhani A, Mittal RD, Mittal B. Microsatellite instability as prognostic marker in bladder tumors: a clinical significance. BMC Urol. 2005. 5:2.
21. Roupret M, Coulet F, Azzouzi AR, Fromont G, Cussenot O. Accuracy of the routine detection of mutation in mismatch repair genes in patients with susceptibility to hereditary upper urinary tract transitional cell carcinoma. BJU Int. 2005. 96:149–151.
22. Roupret M, Fromont G, Azzouzi AR, Catto JW, Vallancien G, Hamdy FC, et al. Microsatellite instability as predictor of survival in patients with invasive upper urinary tract transitional cell carcinoma. Urology. 2005. 65:1233–1237.
23. Amira N, Rivet J, Soliman H, Cancel-Tassin G, Le Duc A, Janin A, et al. Microsatellite instability in urothelial carcinoma of the upper urinary tract. J Urol. 2003. 170:1151–1154.
24. Jiricny J, Nystrom-Lahti M. Mismatch repair defects in cancer. Curr Opin Genet Dev. 2000. 10:157–161.
25. Muller A, Fishel R. Mismatch repair and the hereditary nonpolyposis colorectal cancer syndrome (HNPCC). Cancer Invest. 2002. 20:102–109.
26. Westra JL, Schaapveld M, Hollema H, de Boer JP, Kraak MM, de Jong D, et al. Determination of TP53 mutation is more relevant than microsatellite instability status for the prediction of disease-free survival in adjuvant-treated stage III colon cancer patients. J Clin Oncol. 2005. 23:5635–5643.
27. Myong NH. Thyroid transcription factor-1 (TTF-1) expression in human lung carcinomas: its prognostic implication and relationship with expressions of p53 and Ki-67 proteins. J Korean Med Sci. 2003. 18:494–500.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download