Abstract
Purpose:
The popularity of a partial nephrectomy has grown as a consequence of the increased detection of small incidental renal masses. Herein, our experience of laparoscopic partial nephrectomies is reported.
Materials and Methods:
Between December 2003 and April 2006, 27 cases underwent a laparoscopic partial nephrectomy for renal tumors up to 4cm in diameter. The tumors, and an approximate 0.5cm margin around the tumors, were resected with cold scissors. Hemostasis was achieved with freehand suturing of the pelvocalyceal system and renal parenchyme, over the surgical bolster, using fibrin glue.
Results:
Transperitoneal and retroperitoneal approaches were chosen in 14 and 13 cases, respectively. Hilar clamping of small exophytic tumors was performed in all but 3 cases, with minimal parenchymal invasion. The mean renal tumor size was 2.5cm (ranging from 1 to 4cm). The mean operative and warm ischemia times, and blood loss were 193 minutes (ranging from 115 to 300) and 27.8 minutes (ranging from 15 to 43), and 493ml (ranging from 32 to 1,248), respectively. The mean hospitalization stay was 5.2 days (ranging from 3 to 8 days). Conversion to a laparoscopic radical nephrectomy was required in one case due to a positive frozen biopsy of the resection bed. There were no perioperative complications or open conversions. Histological examinations yielded a renal cell carcinoma in 20 cases, an angiomyolipoma or oncocytoma in 2 cases each, a lipoma in 1 and a leiomyosarcom in 2 cases, two of which had positive margins. One patient underwent selective angioembolization for an asymptomatic renal artery pseudoaneurysm three months postoperatively. All patients were alive, without any local recurrence or metastatic disease, at a mean follow up of 11.4 months (ranging from 3 to 24 months).
REFERENCES
1.Clayman RV., Kavoussi LR., Soper NJ., Dierks SM., Meretyk S., Darcy MD, et al. Laparoscopic nephrectomy: initial case report. J Urol. 1991. 146:278–82.

2.Lightfoot N., Conlon M., Kreiger N., Bissett R., Desai M., Warde P, et al. Impact of noninvasive imaging on increased incidental detection of renal cell carcinoma. Eur Urol. 2000. 37:521–7.

3.Hankey BF., Feuer EJ., Clegg LX., Hayes RB., Legler JM., Prorok PC, et al. Cancer surveillance series: interpreting trends in prostate cancer part I: evidence of the effects of screening in recent prostate cancer incidence, mortality, and survival rates. J Natl Cancer Inst. 1999. 91:1017–24.
4.McKieman J., Simmons R., Katz J., Russo P. Natural history of chronic renal insufficiency after partial and radical nephrectomy. Urology. 2002. 59:816–20.

5.McDougall EM., Clayman RV., Anderson K. Laparoscopic wedge resection of a renal tumor: initial experience. J Laparoendosc Surg. 1993. 3:577–81.

6.Moinzadeh A., Gill IS., Finelli A., Kaouk J., Desai M. Laparoscopic partial nephrectomy: 3-year followup. J Urol. 2006. 175:459–62.

7.Gaur DD. Laparoscopic operative retroperitoneoscopy: use of a new device. J Urol. 1992. 148:1137–9.

8.Guillonneau B., Bermudez H., Gholami S., El Fettouh H., Gupta R., Adomo Rosa J, et al. Laparoscopic partial nephrectomy for renal tumor: single center experience comparing clamping and no clamping techniques of the renal vasculature. J Urol. 2003. 169:483–6.
9.Singh D., Rubenstein M., Gill IS. Laparoscopic partial nephrec tomy. J Endourol. 2005. 19:451–5.
10.Novick AC. Surgery of die kidney. Walsh PC, Retik AB, Vaughan ED, Wein AJ, editors. editors.Campbell' s urology. 8th ed.Philadelphia: Saunders;2002. p. 3602–13.
11.Finelli A., Gill IS. Laparoscopic partial nephrectomy: contemporary technique and results. Urol Oncol. 2004. 22:139–44.

12.Nguyen TT., Parkinson JP., Kuehn DM., Winfield HN. Technique for ensuring negative surgical margins during laparoscopic partial nephrectomy. J Endourol. 2005. 19:410–5.

13.Timsit MO., Bazin JP., Thiounn N., Fontaine E., Chretien Y., Dufour B, et al. Prospective study of safety margins in partial nephrectomy: intraoperative assessment and contribution of frozen section analysis. Urology. 2006. 67:923–6.

14.Zucchi A., Mearini L., Mearini E., Costantini E., Vivacqua C., Porena M. Renal cell carcinoma: histological findings on surgical margins after nephron sparing surgery. J Urol. 2003. 169:905–8.

15.Li QL., Guan HW., Zhang QP., Zhang LZ., Wang FP., Liu YJ. Optimal margin in nephron-sparing surgery for renal cell carcinoma 4cm or less. Eur Urol. 2003. 44:448–51.
16.Castilla EA., Liou LS., Abrahams NA., Fergany A., Rybicki LA., Myles J, et al. Prognostic importance of resection margin width after nephron-sparing surgery for renal cell carcinoma. Urology. 2002. 60:993–7.

18.Dimarco DS., Lohse CM., Zincke H., Cheville JC., Blute ML. Long-term survival of patients with unilateral sporadic multifocal renal cell carcinoma according to histologic subtype compared with patients with solitary tumors after radical nephrectomy. Urology. 2004. 64:462–7.

19.Van PH. Nephron sparing surgery in renal cell carcinoma. Braz J Urol. 2000. 26:342–53.
20.Orvieto MA., Chien GW., Laven B., Rapp DE., Sokolo伴Μ Η., Shalhav AL. Eliminating knot tying during warm ischemia time for laparoscopic partial nephrectomy. J Urol. 2004. 172:2292–5.

21.Albani JM., Novick AC. Renal artery pseudoaneurysm after partial nephrectomy: three case reports and a literature review. Urology. 2003. 62:227.

22.Gill IS., Abreu SC., Desai MM., Steinberg AP., Ramani AP., Ng C, et al. Laparoscopic ice slush renal hypothermia for partial nephrectomy: the initial experience. J Urol. 2003. 170:52–6.

Fig. 1.
A straight line is drawn from the renal hilum to the lateral border of the kidney. Any tumor falling anterior to this line is approached transperitoneally (A) whereas, any tumor posterior to this line is resected through a retroperitoneal approach (B). If the line transgresses the tumor, an approach transperitoneal is used.
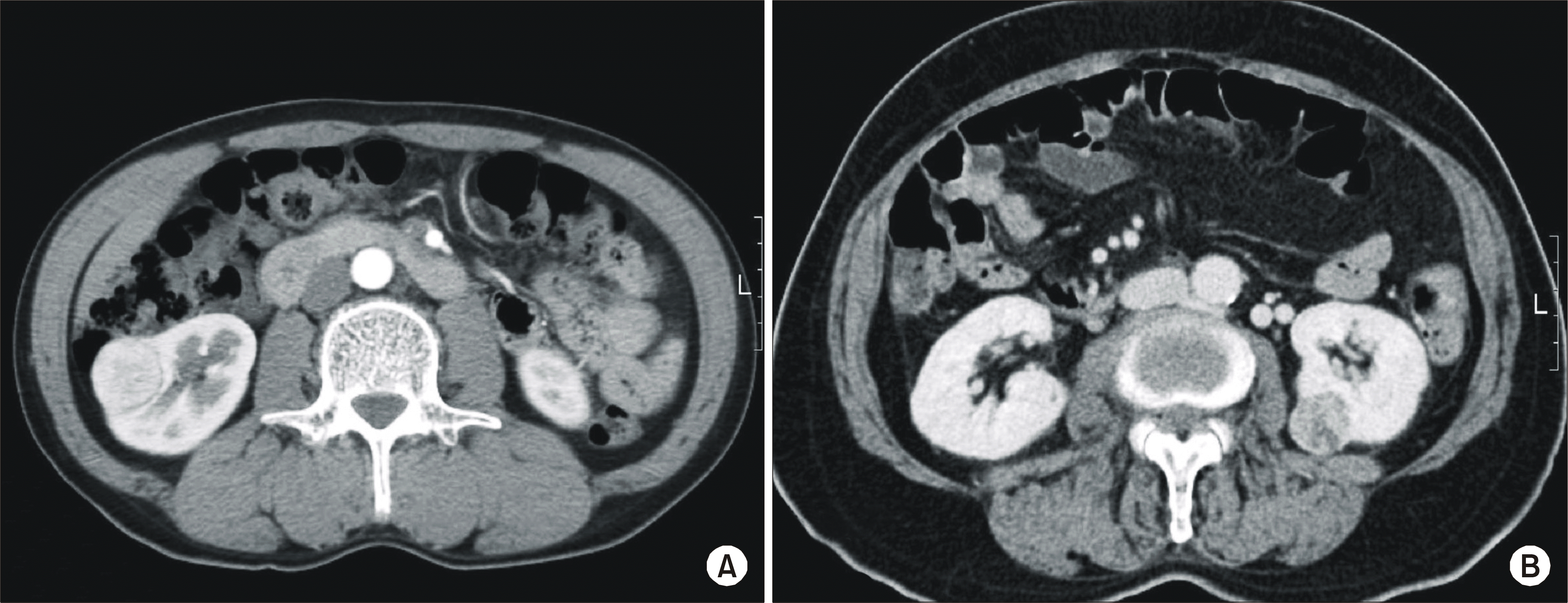
Fig. 2.
(A) Transperitoneal approach. (B) Retroperitoneal approach. ᄋ: 10mm camera port, ·æ additional 10mm port.
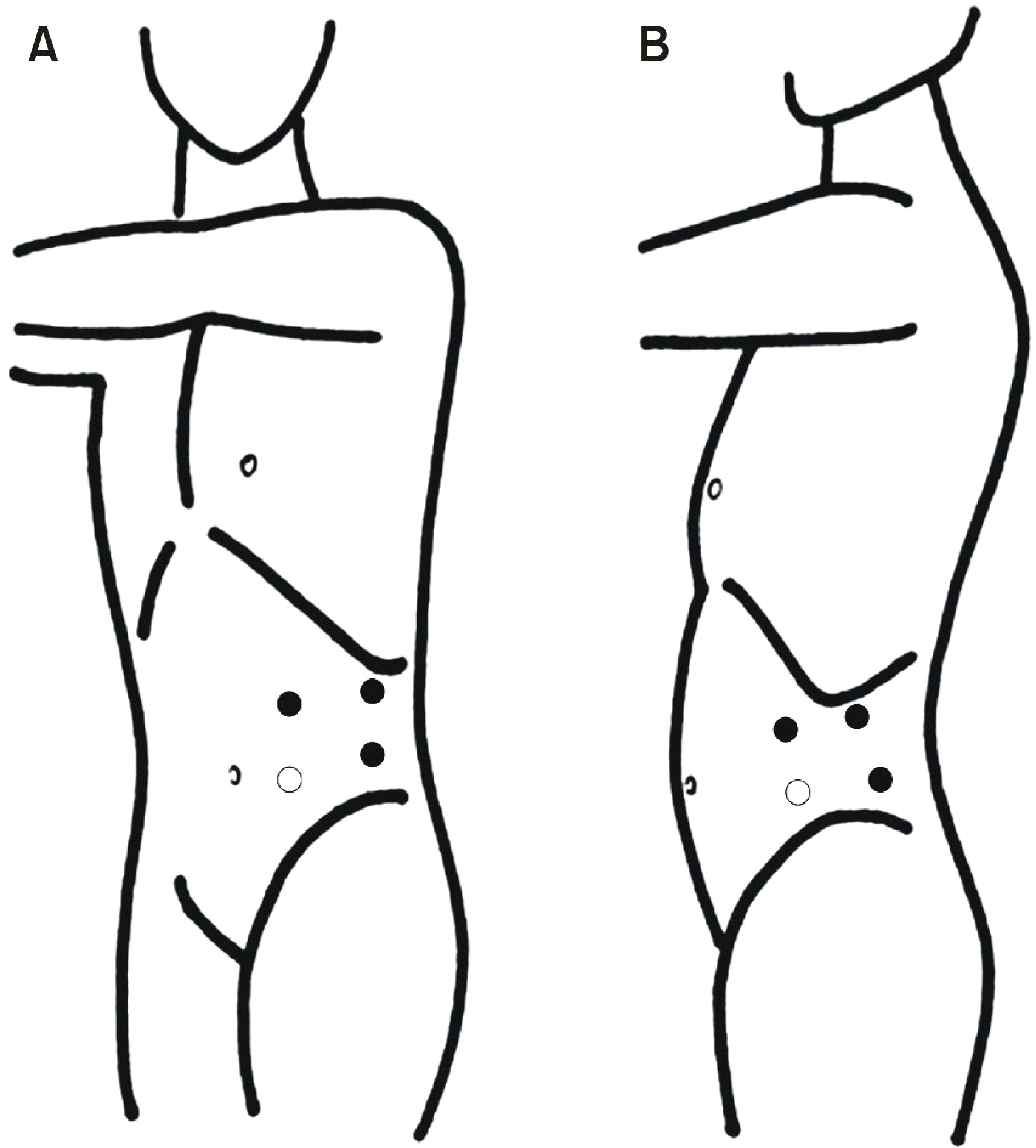
Fig. 3.
(A) The tumor is resected with cold scissors, with an approximate 0.5cm margin maintained around the tumor. (B) A renal parenchymal defect is approximated with freehand suturing, over the surgical bolster, using fibrin glue.
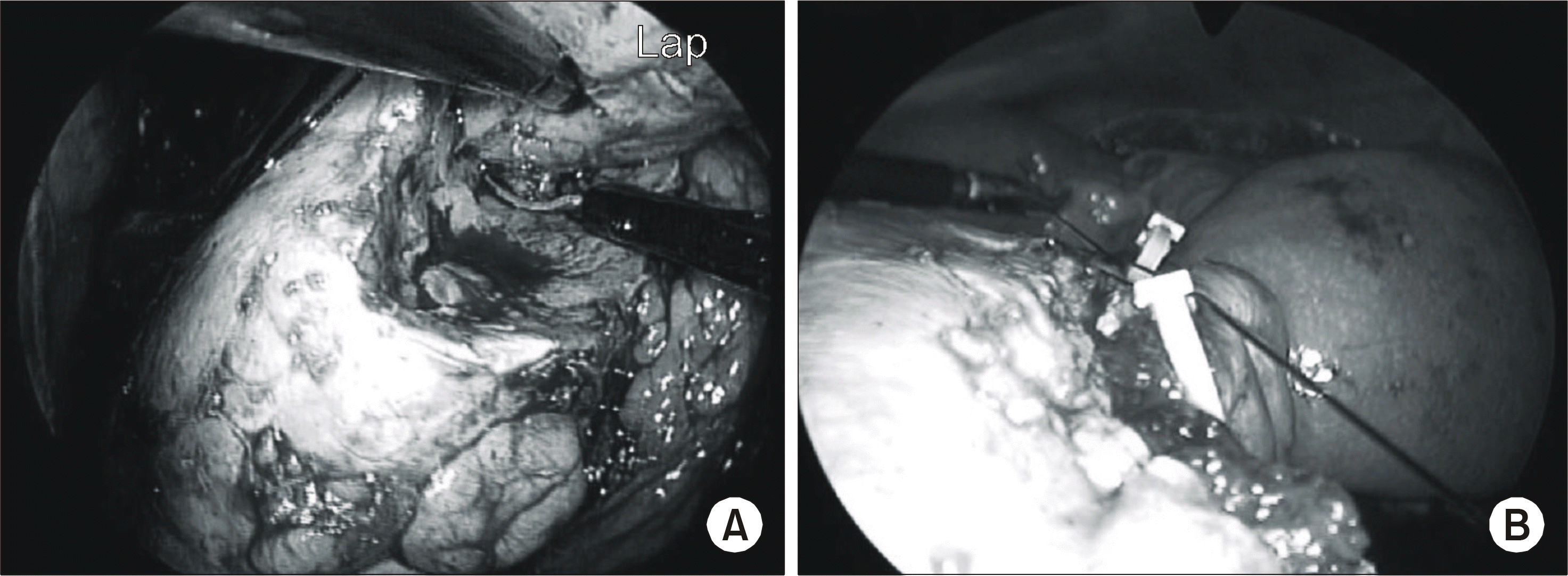
Fig. 4.
(A) DMSA scan shows a focal photon defect due to a partial nephrectomy in the lower pole of the right renal cortex and medulla. (B) The uptake rates are 19.3% and 13.4% (0.014619%/pixel and 0.009962%/pixel) for the left and right kidneys, respectively. DMSA scan: 99mTc-dimercaptosuccinic acid scintigraphy.
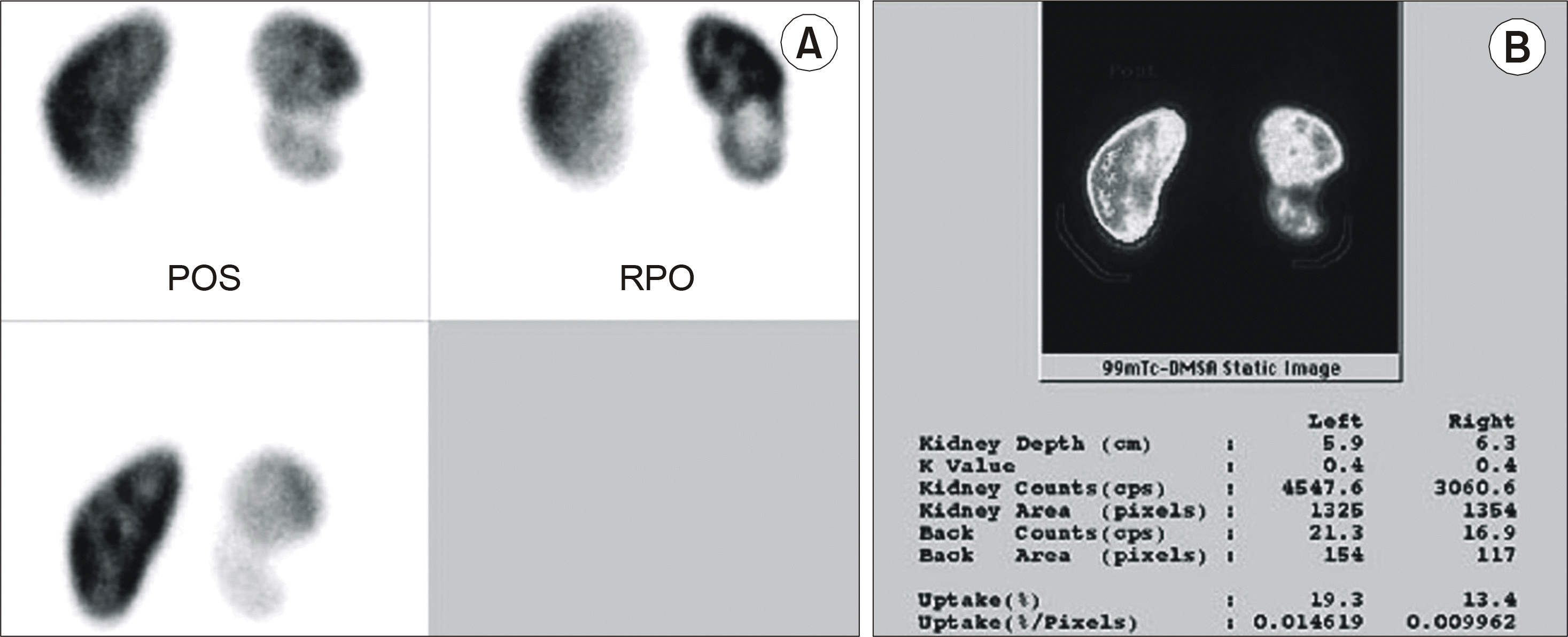
Table 1.
The demographic data on 27 patients
Table 2.
Operative data




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download