Abstract
Purpose
To evaluate regional differences in relative metabolite ratios in the normal human brain by Ή MR spectroscopy (MRS), and compare the spectral quality obtained by the automated prescan method (PROBE) and the manual method.
Materials and Methods
Localized 1H MRS was performed on a GE 1.5 T SIGNA MRl/MRS system (version 5.5) with active shielded gradients. For 20 normal volunteers aged 8 — 47 years, spectral parameters were adjusted by the auto- prescan routine provided by a PROBE package (N二34) and manually(N=33). Five regions of the human brain were examined(N=PROBE, manual): frontal white matter(N=6, 10), parietal white matter(N=8, 9), basal ganglia(N=6, 5), thalamus (N二4, 5), and cerebellum(N=4, 4). For all spectra, a STEAM localization sequence with three-pulse CHESS H2O suppression was used, with the following acquisition parameters :TR=3.0 sec, TE=30 msec, TM=13, 7 msec, SW=2500Hz, SI=2048 pts, AVG=48, and NEX=2.
Results
A total of 61 reliable spectra were obtained by PROBE (28/34=82% success) and by the manual method(33/33= 100% success). Regional differences in the spectral patterns of the five regions were clearly demonstrated by both PROBE and the manual methods. For prescanning, the manual method took slightly longer than PROBE(3 —5 mins and 2 mins, respectively). There were no significant differences in spectral patterns and relative metabolic ratios between the two methods. However, auto-prescan by PROBE seemed to be very vulnerable to slight movement by patients, and in three cases, an acceptable spectrum was thus not obtained.
Conclusion
PROBE is a highly practical and reliable method for single voxel 1H MRS of the human brain å the two methods of prescanning do not result in significantly different spectral patterns and the relative metabolite ratios. PROBE, however, is vulnerable to slight movement by patients, and if the success rate for obtaining quality spectra is to be increased, regardless of the patient's condition and the region of the brain, it must be used in conjunction with the manual method.
Go to : 
REFERENCES
1.Wang Ζ., Zimmerman RA., Sauter R. Proton MR Spectroscopy of the brain: Clinically Usefull information obtained in assessing CNS diseases in children. AJR. 1996. 167:191–199.
2.Tien RD., Lau PH., Smith JS., Lazeyras F. Singlevox el proton brain spectroscopy exam (PROBE/SV) in patients with primary brain tumors. AJR. 1996. 167:201–209.
3.Miller BL., Moats RA., Shonk T., Ernest T., Woolley S., Ross BR. Alzheimer Disease: Depiction of increased cerebral myo-Inositol with proton MR spectroscopy. Radiology. 1993. 187:433–437.

4.Kuhl CK., Layer G., Traber F., Zierz S., Block W., Reiser M. Mitochondrial encephalomyopathy: Correlation of P-31 exercise MR spectroscopy with clinical findings. Radiology. 1994. 192:223–230.

5.Song IC., Chang KH., Han MH, et al. In vivo single voxel Ή spectroscopy in cerebral glioma. J Korean Radiol Soc. 1996. 35:307–314.
6.Lanfermann H., Kugel H., Heindel W., Herholz K., Heiss W-D., Lackner K. Metabolic changes in acute and subacute cerebral infarctions: Findings at proton MR spectroscopic imaging. Radiology. 1995. 196:203–210.

7.Ross RD., Jacobson S., Villamil F, et al. Subclinical hepatic encephalopathy: proton MR spectroscopic abnormaities. Radiology. 1994. 193:457–463.
8.Rajanayagam V., Grad J., Krivit W, et al. Proton MR spectroscopy of childhood adrenoleukodystrophy. AJNR. 1996. 17:1013–1024.
9.Chang KH., Jeon BS., Song IC, et al. Ή MR spectroscopy in Parkinson's disease and progressive supranuclear palsy: Preliminary stydy. J Korean Radiol Soc. 1996. 34:711–716.
10.Chong WK., Sweeney B., Wilkinson ID, et al. Proton spectroscopy of the brain in HIV infection: Correlation with clinical, immunologic, and MR imaging findings, Radiology. 1993. 188:119–124.
11.Shonk TK., Mosts RA., Gifford P, et al. Probable Alzheimer disease: Diagnosis with proton MR spectroscopy. Radiology. 1995. 195:65–72.

12.Tzika A A., Ball WS Jr., Vigneron DB., Dunn RS., Nelson SJ. Kirks D. Childhood adrenoleukodystropy: Assessment with proton MR spectroscopy. Radology. 1993. 189:467–480.
13.Ott D., Hennig J., Ernst T. Human brain tumors: Assessment with in vivo proton MR spectroscopy. Radiology. 1993. 186:745–752.

14.Bizzi A., Movsas B., Tedeschi G, et al. Response of Non-Hodgkin lymphoma to radiation therapy: Early and long-term assessment with Ή MR spectroscopic imaging. Radiology. 1995. 194:271–276.
15.Knaap MS., Grond J., Rijen PC., Faber JAJ., Valk J., Willemse K. Age-dependent changes in localized proton and phosphorus MR spectroscopy of the brain. Radiology. 1990. 176:509–515.

16.Kimura H., Fujii Y., Itoh S, et al. Metabolic alterations in neonate and infant brain during development: Evaluation with proton MR spectroscopy. Radiology. 1995. 194:483–489.
17.Kreis R., Ernst T., Ross BD. Development of the human brain: In vivo quantification of metabolic and water content with proton magnetic resonance spectroscopy. MR Μ. 1993. 30:424–437.
18.Webb PG., Sailasuta N., Kohler SJ., Raidy T., Moats RA., Hurd RE. Automated singlevoxel proton MRS: technical development and multisite verification. Magn Re son Med. 1994. 31:365–373.

19.Jung Hee Lee., Choong Gon Choi., Sang-Tae Kim, et al. Localized single voxel Ή MR spectroscopy toward routine clinical use. J Korean Radiol Soc. 1996. 34:185–191.
20.In Chan Song., Kee Hyun Chang., Kwan Hong Min, et al. ΧΗMR spectroscopic patterns of normal adult brain. J Korean Radiol Soc. 1996. 35:435–440.
21.Moats TA., Watson L., Shonl T, et al. Added value of automated clinical proton MR spectroscopy of the brain. J Comput Assist Tomogr. 1995. 19:480–491.

23.Βrun H., Michaelis T., Merboldt KD, et al. On the interpretation of proton NMR spectra from brain tumors in vivo and in vitro. NMR Biomed. 1992. 5:253–258.
Go to : 
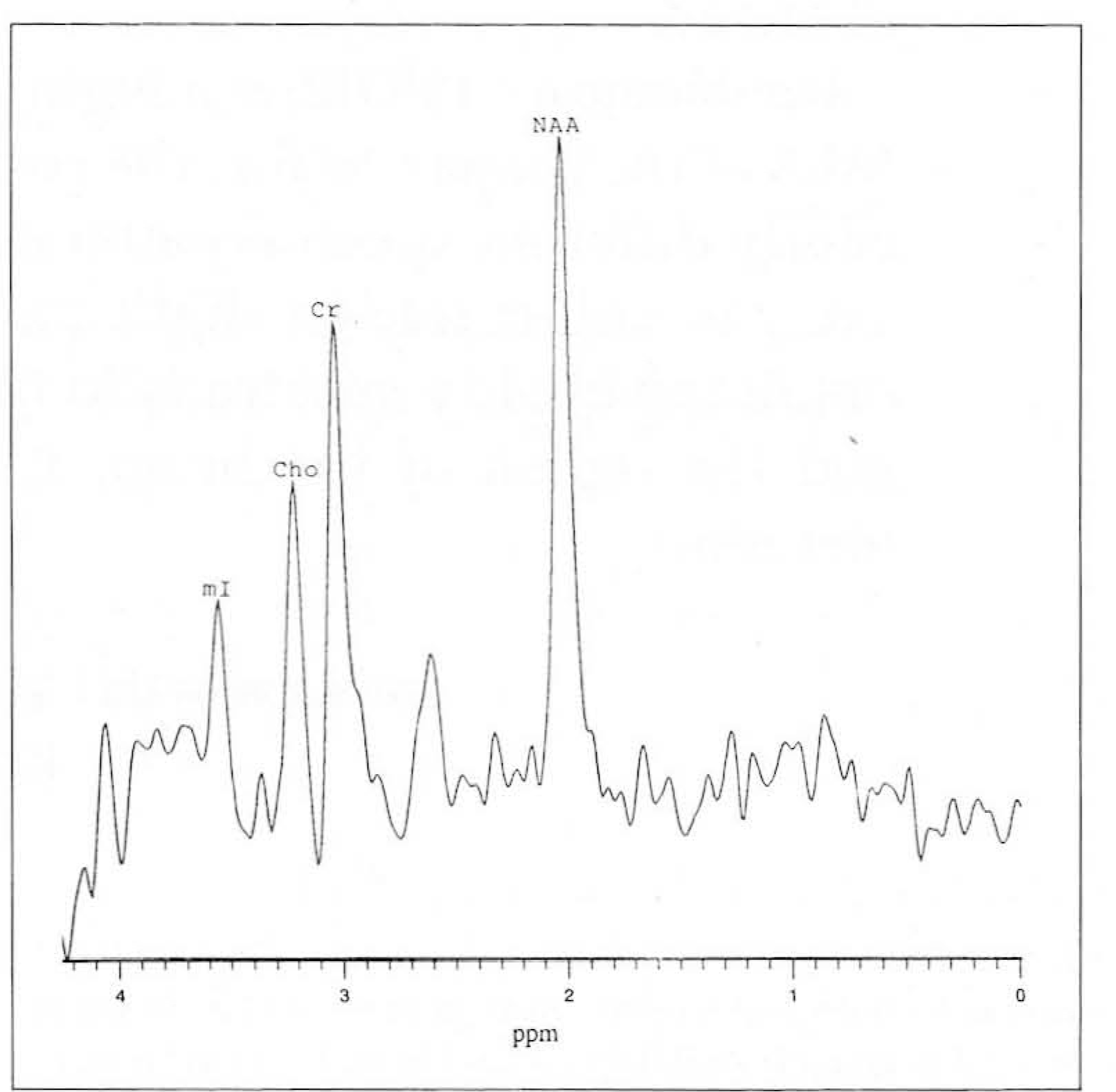 | Fig. 1.
1 H MR spectroscopy of basal ganglia in 24 year-old woman with PROBE methods. NAA : N-acetylaspartate, Cr : creatine/phosphocreatine Cho : choline compounds, mI : myo-inositol |
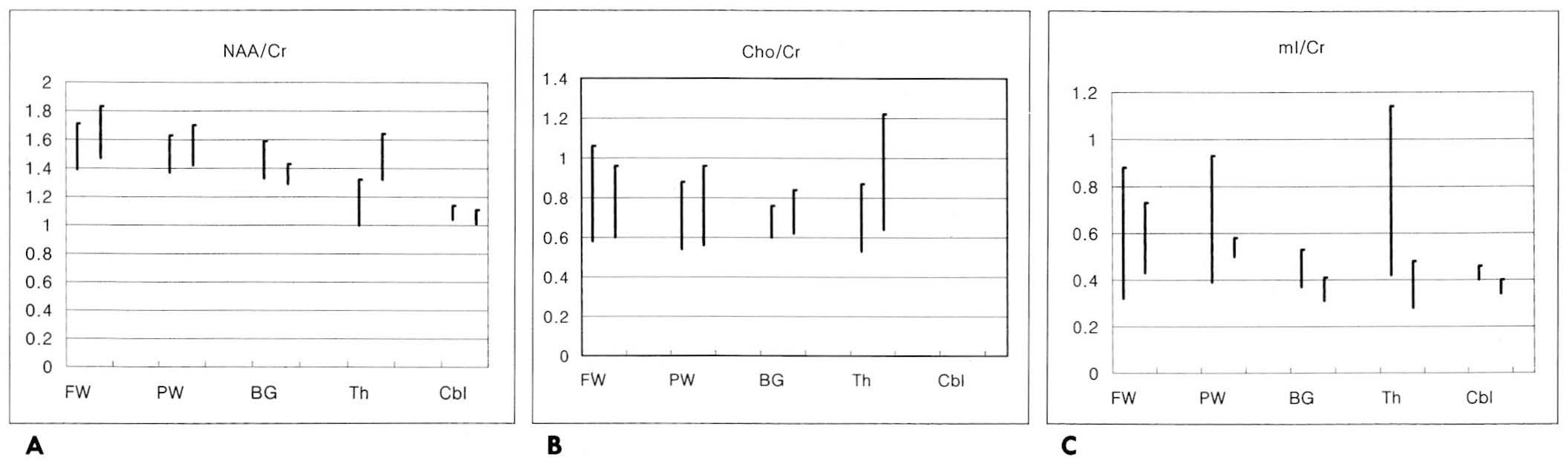 | Fig. 2.Graphs of the relative metabolite ratio with PROBE and manual method in the various sites of the brain A. NAA/Cr, B. Cho/Cr, and C. ml/Cr FW : frontal white matter, PW : parietal white matter, BG : basal ganglia, Th : thalamus, Cbl: cerebellum NAA : N-acetylaspartate, Cr : creatine / phosphocreatine, Cho : choline compounds, ml: myo-inositol |
 | Fig. 3.Ή MR spectroscopy of the various cerebral regions with both PROBE (1) and manual method (2). There are no significant differences of the spectral patterns in the various sites of the brain. A. Frontal white matter, B. Parietal white matter, C. Basal ganglia, D. Thalamus, and E. Cerebellum NAA : N-acetylaspartate, Cr: creatine /phosphocreatine Cho: choline compounds, ml: myo-inositol |
Table 1.
Relative Metabolite Ratio with PROBE/SV
Table 2.
Relative Metabolite Ratio with Manual Method




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download