Abstract
Systemic lupus erythematous (SLE) is a systemic autoimmune disease with multi-organ inflammation caused by the production of pathogenic autoantibodies and immune complexes reflecting a global loss of tolerance. Lupus nephritis (LN) is present in approximately 60% of SLE patients and is considered a major predictor of a poor prognosis. To date, many studies utilizing genomics, transcriptomics, epigenetics, metabolomics, and microbiome have been conducted on a range of animal models and lupus patients to understand the pathogenesis of SLE and LN. Collectively, these studies support the concept that LN is caused by increased cell death, which has not been properly dealt with; abnormal innate immunity; hyperactive adaptive immunity; and genetic variants triggered by a range of environmental factors. This review summarizes the results from studies that contributed strongly to elucidating the pathogenesis of SLE and LN, highlighting the immunological and non-immunological mechanisms.
Lupus nephritis (LN) is a representative clinical feature of systemic lupus erythematous (SLE), which is scientifically challenging to comprehend its nature. The pathogenesis of LN involves a variety of pathogenic mechanisms. The pathogenesis of LN implicates altered cell death including aberrant apoptosis and formation of neutrophil extracellular traps (NETs) in breaking tolerance, the significance of autoantibodies, the role of the complement cascade, the contributions of adaptive immunity cross-talked with the innate immune system, genetic associations and various environmental factors in driving renal damage (Figure 1).
In spite of a knotted skein slowly being unraveled in return for endless efforts of researchers in various fields, we still haven't figured out the exact cause, which prevents us from achieving the goal of cure. In addition, some results have not been repeated in following studies and not been validated yet. For example, the intrarenal etiology of LN includes the several paradigms contemporarily in conflict with each other such as anti-double strand DNA (dsDNA) antibodies that cross-react with inherent renal antigens, anti-dsDNA antibodies targeting exposed chromatin in glomeruli, and relative antibody avidity for dsDNA, chromatin fragments, or cross-reacting antigens. In addition, LN patients have been reported to have increased numbers of apoptotic glomerular cells compared to healthy controls, in correlation with anti-dsDNA antibody levels, complement consumption, and cell proliferation while there are also evidences about a decrease in apoptotic cells from the glomerulus and tubulo-interstitium in LN biopsies compared to control kidneys. Therefore, I'd like to suggest that you stop here if you are eager to find the right answer to the pathogenesis of LN through this review. And for those who are only interested in LN rather than the whole story of SLE, the pathogenesis specific to LN is written in Italics.
SLE is a systemic autoimmune disease with multi-organ inflammation by production of pathogenic autoantibodies directed against nucleic acids and their binding proteins and immune complexes reflecting a global loss of tolerance [1]. The prevalence of SLE ranges from 1.4% to 21.9%; incidence is estimated to be 7.4~159.4 cases per 100,000 person-years [2]. In Korea, the prevalence was reported to be around 20 per 100,000 populations and there are approximately 12,000 patients under treatment [3]. Most patients are female and younger than 50 years of age. However, male patients have a high incidence of nephropathy and greater severity of disease [4]. With the advent of advanced therapies, the 5-year survival rate has shown continuous improvement from 50% in 1953~1969 to nearly 90% to date [56].
LN is present in approximately 60% of SLE patients, with 25%~50% of patients presenting with clinical renal disease at the time of diagnosis [7], when occurring early in the course of SLE, is considered a major predictor of poor prognosis [8]. Patients with LN also have a higher standardized mortality ratio (6~6.8 vs. 2.4) and die earlier than SLE patients without LN [9101112].
Accelerated cell death in SLE can potentially overwhelm the host clearance mechanism, resulting in the accumulation of apoptotic debris. And these changes contribute to induction of autoantibodies and other aberrant immune responses in SLE and in LN specifically [13]. Secondary necrotic cells release nuclear autoantigens that can lead to immune complex formation (Figure 2).
Some apoptotic signaling molecules have been reported to be related to SLE. One of these was the identification of mutations in Fas receptor and Fas ligand in mice [141516] and in humans that develop autoimmune lymphoproliferative syndrome (ALPS) [1718].
Defects in phagocytosis have been observed in SLE [19]. SLE patients have an accumulation of apoptotic cells in lymph node germinal centers likely due to the reduction in tangible body macrophages that specialize in the removal of dead cells [19]. Defects in the differentiation of myeloid progenitors into macrophages may potentially lead to phagocytosis defects in SLE [20].
Apoptosis-induced post-translational histone modifications are targets for autoimmune system in SLE [212223]. Microparticles from SLE patients which contain apoptosis-related histone modifications activate plasmacytoid dendritic cells (pDCs) and myeloid DCs (mDCs) that results in the induction of proinflammatory cytokines such as type I interferon (IFN) [24].
Some apoptotic signaling molecules including B cell lymphoma 2 (Bcl-2), Bim, transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI), B cell-activating factor (BAFF), phosphatase and tensin homolog (PTEN), and p53 have also been linked to LN [25].
Whether increased cell death of glomerular cells is an important source of circulating and/or tissue nucleosomes promoting glomerulonephritis is controversial [26]. LN patients have been reported to have increased numbers of apoptotic glomerular cells compared to healthy controls, in correlation with anti-dsDNA antibody levels, complement consumption, and cell proliferation [27]. But, there are also evidences about a decrease in apoptotic cells from the glomerulus and tubulo-interstitium in LN biopsies compared to control kidneys [28]. In addition, renal cells from LN patients had enhanced proliferation without an increase in apoptosis. It is not clear why there are significant inconsistencies in determining if renal cells from LN patients undergo increased apoptosis, but one potential explanation could be the type of experimental method used to quantify this process [2930]. On the other hand, the deposition of glomerular ubiquitinated histone H2A was reported in a significant proportion of LN [3132].
Enhanced formation and defective clearance of NETs contributes to SLE, especially renal disease (Figure 2). Neutrophils can extrude a meshwork of nuclear material bound to neutrophil granular proteins which mediates cleaving histones and promoting chromatin decondensation [33]. NET induction and clearance may result in a protective antimicrobial effect but excessive NET formation and inefficient removal could lead to tissue damage and autoantigen modification and externalization [34]. SLE patients are more prone to form NETs than neutrophils from healthy controls [35363738]. And SLE patients have an impaired ability to degrade NETs and proposed that this impairment contributes to the development of LN [39404142]. NET derived self-DNA complexed with neutrophil-derived antimicrobial peptides activatepDC Toll-like receptor (TLR) 9 and induce IFN α [38].
The renal biopsy analysis from patients with LN revealed the presence of NETs and infiltrating netting neutrophils in the glomeruli [35], which positively correlates with higher levels of circulating autoantibodies and enhanced activity index in kidney biopsies. Deoxyribonuclease (DNase) I is the major endonuclease found in circulation involved in degrading NETs. The correlation between DNase I deficiency and increased prevalence of LN was confirmed in SLE patients with renal involvement [394143].
TLR: Persistent apoptotic debris containing nucleic acids can stimulate the inflammatory response through the activation of nucleic acid recognition receptors such as members of the TLR [44]. In SLE, TLRs might become aberrantly activated in the absence of foreign molecules [45] and associated with pDC activation and type I IFN production (Figure 3) [3638]. Indeed, TLR7 (receptor for single strand RNA) and TLR9 (receptor for DNA) mRNA expression was upregulated in PBMCs from SLE patients and levels correlate with the expression of IFN α [4647]. TLR7 was preferentially increased in SLE patients with antibodies against RNA-associated antigens, while TLR9 induction correlated with anti-dsDNA antibody titers [48]. And the upregulation of TLR7 was observed when healthy neutrophils were cultured with sera from SLE patients with active disease [36]. SLE patients with active disease had a higher number of TLR9 expressing B cells and monocytes than did patients with low disease activity, and levels of these cells correlated with levels of antibodies to dsDNA [49]. In TLR9 deficient lupus-prone mice, the generation of anti-dsDNA and anti- chromatin autoantibodies was specifically inhibited [50].
The nucleic acid component of immune complexes also activates intrarenal inflammation by TLRs in intrarenal macrophages and DCs to produce large amounts of proinflammatory cytokines and IFN [5152535455565758]. In pristane-treated mice, TLR7 was specifically required for the production of RNA-reactive autoantibodies and for the development of glomerulonephritis [59]. Studies of pharmacologic or genetic manipulation of TLR7 expression or function support a central role for TLR7 in inflammation, loss of tolerance, and type I IFN production [60616263]. And the activation of TLR3 on antigen presenting cells (APCs) or renal mesangial cells can aggravate LN by recruiting polymorphonuclear cells to the site of inflammation, where they can contribute to renal injury [6465]. Genetic variants of TLR3 (receptor for dsRNA), TLR7/8 and TLR9 have been associated with LN. These variants may contribute to severe renal insufficiency in LN. In addition, signaling through particular TLR9 genetic variants was associated to more severe renal disease at the time of LN presentation [6667].
Cytokines: Levels of many cytokines are elevated in SLE such as IFN, TNF, interleukin (IL)-4, IL-6, and IL-10 and their main effects are the promotion of autoantibody production and inflammation (Figure 3).
Type I and II IFNs have emerged as key cytokines in the pathogenesis of SLE and increases in their levels precede autoantibody development [68]. Upregulation of TNF can increase type I IFN expression [6970]. IFN α, a type I IFN has multiple effects consistent with known immunologic features of SLE, such as upregulation of BAFF, decreased regulatory T (Treg) cell function, and induction of plasma cells. In particular, the prevalence of type I IFN signaling was higher in T cells than in other immune cell types in patients with SLE [71]. A direct pathogenic role for IFN in mouse models of lupus is also supported by studies in which exogenous administration of IFN α exacerbates disease [7273].
Patients with SLE may also have an imbalanced T cell cytokine profile characterized by decreased IL-2 [74] and increased IL-17 levels [75]. Production of IL-2 is impaired on multiple levels [74]. IL-2, in addition to being critical for Treg cell development and function, is also necessary for restricting expression of IL-17. In SLE, IL-17 may mediate local tissue damage through the induction of inflammatory cytokines and chemokines, and by recruiting other immune cells. The differentiation of the T helper cell subset producing IL-17 is dependent on IL-23, and an anti-IL-23 antibody ameliorated disease in one mouse model of lupus [76].
B cell activation and autoantibody production are promoted in SLE by BAFF. Serum levels of BAFF are increased in patients with SLE and positively correlate with autoantibody titers [77]. Transgenic overexpression of BAFF in a mouse model of lupus exacerbated disease [78], BAFF is a critical factor for B cell homeostasis and high BAFF levels might reduce the stringency of B cell selection, allowing autoreactive clones to persist in the periphery [79].
Following immune complex deposition, a large variety of inflammatory mediators is produced in LN kidneys with spreading of the response as disease progresses [8081]. A Type I IFN signature is also a feature of LN kidneys [8283]. IFN has multiple detrimental effects on the kidneys including vascular rarefaction and injury to glomerular parietal cells and podocytes [8485]. Examples include CCL2, a chemokine expressed early in the glomerulonephritis process, and TNF that is expressed at proteinuria onset [8687]. Multiple cytokines such as IFN γ, IL-21 and IL-17 have also been detected in LN kidneys [88]. Once tissue injury occurs, soluble products released from injured cells amplify the inflammatory response by stimulating extracellular and intracellular innate immune receptors [8990919293]. Nevertheless, not all renal inflammatory mediators are necessary for the inflammatory process. For example, IL-17 deficiency alters the course of LN only in some models in which Th17 cells infiltrate the kidneys [9495]. IL-4 drives signal transducer and activator of transcription 4 (STAT4) activation, which leads to autoantibody production. Autoantibody-mediated pathology in LN is supported further by genetic variants within the T follicular helper (Tfh) differentiation pathway [9697], where IL-6 and IFN γ [98] via STAT [99] activate TfH differentiation.
Complements: The complement systems affect the ability of innate immune cells to facilitate phagocytosis of nuclear antigens and cell debris. One of the most remarkable genetic associations in SLE is the early components of the complement system classical pathway [100101102103]. More than 90% of patients with homozygous deficiency of C1q are reported to have SLE and the high titers of autoantibodies are observed in more than 70% of these patients [100101102104]. About 10%~30% of homozygous C2-deficient patients develop SLE [100105106]. Arthritis, malar rash, discoid rash, and photosensitivity are seen in the majority of C2-deficient patients with SLE [100, 101103104106]. Complete homozygous deficiency of C4 is rare but, more than 75% of these patients develop this disease. Approximately 50% of SLE-C4-deficient patients develop LN and more than 70% has antinuclear antibodies and anti-Ro autoantibodies in their serum [100101104].
Patients with C1 deficiencies usually present SLE at an early age, in similar female:male proportions, with severe symptoms and prominent cutaneous manifestations [107]. Anti-C1q antibodies which target a neo-epitope of bound C1q are present in 2%~8% of the healthy population, but they are present in 30%~48% of patients with SLE [108]. Their titer correlates to active renal disease with a sensitivity of 44%~100% and a specificity of 70%~92% [109].
The complement system is generally activated in LN and can directly mediate kidney injury through the terminal pathway, or indirectly increase renal inflammation by recruiting leukocytes to the kidney. In a lupus cohort, 23% of patients had autoantibodies to C1q and to C3b [110]. Anti-C3b and anti-C1q levels tended to increase in the months leading up to renal flare [111]. LN has been associated genetically with deficiencies in the opsonin C1q, C2 and C4 [112], Genetic variants of C-reactive protein (CRP) [113] and mannose binding lectin [114], also contribute to LN by disrupting complete clearance of autoantigens, enhancing inflammation, and increasing autoantibodies to C1q.
B cells: The absolute number of B cells is not different to that of controls in SLE patients. But, certain peripheral B cell subsets in SLE patients showed differently compared to healthy controls by the following mechanisms (Figure 3).
Loss of tolerance and altered B cell differentiation in SLE might present from birth or acquired as part of the disease process [115]. Human studies have clearly implicated loss of B cell tolerance. Early immature B cells show increased levels of autoreactivity in SLE, possibly owing to a break in central B cell tolerance [116]. Patients with inactive SLE fail to remove B cells expressing self-reactive B cell receptors (BCRs) expressed by naïve B cells due to defects of selection against autoreactive B cells [117]. Activation of B cells through the TLR pathway or cytokines such as BAFF promotes loss of tolerance. Mouse models have demonstrated that transitional B cells are susceptible to accelerated maturation by TLR 9, which bypasses tolerance checkpoints [118119120121]. In addition, IL-10 secreting B cells with regulatory capabilities show functional impairment in SLE [122123].
High number of self-reactive mature naïve B cells which subsequently originate autoantibody producing plasma cells is the most reported characteristic of the abnormal B cell homeostasis in SLE characterized by the expansion of peripheral plasmablasts [124], which also correlates with disease activity and the titer of autoantibodies [125]. And the pool of memory B cells is enlarged. Since these cells have low activation thresholds, they present a risk for autoimmunity and the regulation by FcγRIIb receptors may be inhibited [126]. So these cells can be rapidly activated in a non-antigen-specific manner by the combination of TLR agonists and a proliferation-inducing ligand (APRIL) (TNFSF13A) or BAFF (TNFSF13B) as well as by the combination of cytokines, such as IL-21 and BAFF [77127].
Anti-dsDNA antibodies react with several renal cell types and are thought to be central to the nephritis process. The relative amount of anti-dsDNA antibodies has been calculated to comprise up to 20% of the total eluted immunoglobulin (Ig)G from nephritic kidney [128129130131132]. There are two theories about the pathogenic process about dsDNA antibodies. First, anti-dsDNA antibodies recognize exposed chromatin in the mesangium or in glomerular basement membrane. DNA specific B cells are stimulated by chromatin fragments and histone specific T helper cells. The emerging anti-dsDNA antibodies bind exposed chromatin in glomeruli and initiate LN [128133]. The completely lostof renal DNase I during progression of SLE seems to reduceclearance of chromatin from dead cells, and to promote harmful accumulation of undigested chromatin in glomeruli [133134135]. Others indicate that antibodies target cross-reacting antigens that appear as normal constituents in glomeruli [133136] or that chromatin- IgG complexes derive from circulation [137138139]. The B cells specific for chromatin or inherent glomerular structure such as laminin or entactin, respond by producing cross-reactive anti-dsDNA/anti-chromatin antibodies. These antibodies may bind exposed chromatin fragments or homologous, inherent antigens in kidneys, lungs, and other organs [133136]. Autoantibodies to annexin1 and α enolase have also been detected in LN kidneys [140]. Meanwhile, Infiltrating leukocytes form de novo lymphoid organs inside the kidney, which involve the clonal expansion of B cells. Such B cells undergo intrarenal proliferation and activation, which contributes to local inflammation and tissue pathology in addition to their role for systemic and intrarenal autoantibody production [141142]. B cells derived from human LN biopsies recognize vimentin, an intracellular structural protein that is cleaved and extruded from apoptotic cells [143]. Serum anti-vimentin antibodies are associated with decreasing GFR and increasing tubulointerstitial damage, and are associated with severe interstitial disease in LN [143144].
There are genetic variants that affect B-cells to break tolerance, secrete autoantibodies that contribute to kidney damage in LN. Genetic variants in the BCR complex and proximal signaling molecules are enriched in SLE patients and may contribute to LN [145]. The SLE patients with genetic variation of CSK has amplified inhibitory phosphorylation of Lyn, thus increasing BCR-mediated activation of mature B cells [146]. And these patients carries many lupus-associated autoantibodies that contribute to LN [147]. Genetic variants of CD40 which positively regulates B-lymphocyte activation through the adaptor molecule TRAF6 are associated with LN [148]. CD40 synergizes with TLRs and the BCR allowing to further drive immune dysregulation associated with SLE and renal disease in LN [149].
T cells: Loss of T cell tolerance through multiple mechanisms exists in SLE. There is aberrant signaling through the T cell receptor (TCR) in patients with SLE. In T cells from patients with SLE, the CD3ζ chain (which mediates signaling via tyrosine-protein kinase ZAP 70) is down regulated, causing ZAP 70 to be replaced by FcRγ. FcRγ then pairs with tyrosine-protein kinase SYK rather than with ZAP 70, resulting in hyperactivation of the TCR signaling pathway [150151]. Despite this hyperactivated phenotype, T cell production of IL 2 is actually impaired [74152].
Patients with SLE also show altered T cell subset populations (Figure 3). Th17 cells found infiltrating the kidneys of patients with lupus nephritis, and in the skin lesions of patients with SLE [153]. Double-negative T cells (CD4−CD8−) are expanded in patients with SLE [154155] and seem to be the primary source of IL 17 in SLE [156]. And these T cells are thought to contribute to loss of tolerance [154155], as they also express IL 1β and IFN γ, and promote B cell differentiation and antibody production.
Both B cells and T cells from LN kidneys are clonally expanded, and the same T cell expansions have been detected in the peripheral blood [143157] and in the urine of LN patients [158]. Aberrant T cell–B cell interactions are also observed in SLE [159160]. The pathologically expanded and activated TfH cell compartment markedly affects B cell differentiation. And expansion of the TfH cell subset correlates with increased disease activity and severity in patients with SLE [161162163]. The expansion of TfH cells in SLE may be directed by interaction with OX40 ligand (also known as TNF ligand superfamily member 4 [TNFSF4]), which is expressed on myeloid antigen-presenting cells [164]. Genetically determined increased OX40L expression promotes human SLE by effector T cells proliferation and plasma cell development. Loss of B cell OX40L ameliorates the SLE through declining in TfH cell numbers [165].
T cells from LN kidneys are clonally expanded, and the same T cell expansions have been detected in the peripheral blood [143], particularly IL-17 producing CD3+/CD4+ or CD3+CD4/8−/−T cells [156]. And multiple T cell cytokines such as IFN γ, IL-21 and IL-17 have also been detected in LN kidneys [88]. TfH cells can be seen within lymphoid aggregates in kidney biopsy samples from patients with active LN, and activated TfH cells correlate with autoantibody titers in these patients [96166].
Circulation-‘the vessels are tossed and turned’: Endothelial cells produce Platelet-derived growth factor (PDGF)-B whose interaction with PDGF-Rβ on mesangial cells is required for the development of glomerular disease. Expression of PDGF isoforms is upregulated in many forms of renal injury, causing mesangial hyperproliferation, matrix production, cytokine and chemokine release, and renal fibrosis [167]. Podocytes and endothelial cells also interact by bidirectional diffusion of cytokines/growth factors through the glomerular basement membrane [168]. And in diseased tissue, both activated glomerular endothelial cells and damaged podocytes release endothelin 1 that amplifies glomerular injury by causing mitochondrial stress [169].
And there are abnormal vascular function and tissue hypoxia in LN. The capacity for angiogenesis and capillary repair is lost owing to dissociation pericyte from capillaries and diminished production VEGF, leading to capillary infarction in both the glomerulus and the interstitium [170171]. Other disturbances of angiogenesis reported in LN include a decrease in the ratio of pro-angiogenic Ang1/anti-angiogenic Ang2, down regulation of the angiogenic factor FGF-2, an increase in the VEGF inhibitor ADAMTS-1, and alterations in endothelial nitric oxide synthase [172173174175]. Recent studies have shown that injured renal tubular cells have mitochondrial dysfunction, reprogram them to a pro-fibrotic phenotype, and contribute to their death [80]. Fibroblasts may contribute to tissue injury by producing pro-inflammatory mediators [176]. Fibrotic tissue may disrupt normal anatomic structures and interfere with oxygen diffusion, thus exacerbating hypoxia [177].
SLE is known to have a strong genetic link, with a heritability of 66%. Data suggest that concordance of SLE is 10 times more frequent in monozygotic than in dizygotic twins. The twin concordance rate for SLE is 25%~30% in monozygotic twins compared with 2% in dizygotic twins [178]. Most of genes associated with SLE are associated with multiple autoimmune diseases. The overall genetic risks identified to date are limited, with each gene generally conferring a relative risk <2 (Figure 4).
The rare but high-risk deficiencies in complement pathway gene products, including C2, C4, and C1q, are thought to contribute to lupus pathogenesis by impairing clearance of cellular debris [179180]. SLE develops in over 90% of C1q-deficient individuals [181]. Similarly, SLE development is strongly associated with C4 deficiency (75%) and to a lesser degree with homozygous C2 deficiency (10%~30%) [182]. However, the deficiency of these genes in patients with SLE is extremely rare.
Several genes have been associated with SLE susceptibility, most prominently in the human leukocyte antigen (HLA) loci [183184]. Then, FcγRIIA and FcγRIIIB which mediate the phagocytosis and immune function of the immune complex have been reported, In addition, CRP and integrin alpha M (ITGAM) are related [185, 186]. Mutation in the integrin α M (CD11b)-encoding ITGAM gene induced TLR-dependent proinflammatory signaling and IFNα signaling in lupus-prone MRL/Lpr mice [187].
HLA DRB1*1501 (DR2) and DR3 B1*0301 are class II alleles consistently shown to be associated with SLE [188]. More recently, a large genome wide association studies (GWAS) found that the best model for association was a combination of HLA alleles including B*08:01 and B*18:01 in class I, DQB1*02:01,DRB3*02:00, and DQA*01:02 in class II and a class III single nucleotide polymorphism (SNP) (rs74290525) located in SLC44A4 [184189]. Recent GWAS have superseded older candidate gene studies and have shown >40 genes associated with SLE outside of the MHC in European populations [189]. A large number of lupus-associated SNPs found in genes that encode proteins involved in induction of type I IFN and the innate immune response in SLE pathogenesis, such as IFN regulatory factor 5 (IRF5) and IRF7, TNF α-induced protein 3 (TNFAIP3) [190191192193]. And the expression of type I IFN signature genes such as Interferon α-inducible protein 27 (IFI27), interferon-induced protein 44-like (IFI44L), lymphocyte antigen 6 complex, locus E (LY6E), TNFAIP6, and interferon induced transmembrane protein 1 (IFITM1) in SLE patients was increased compared to other patients with autoimmune disease [194]. Additional lupus-associated variants that alter adaptive immune system activation are involved in cytokine signaling, such as STAT4, or efficiency of signaling downstream of the T and B cell surface antigen receptors, such as , protein tyrosine phosphatase, nonreceptor type 22 (PTPN22) in the case of both T and B cells, and LYN, B cell scaffold protein with ankyrin repeats 1 (BANK1), B lymphoid tyrosine kinase (BLK), TNFAIP3, and others in the case of B cells [195].
A GWAS in Koreans has been recently published [196]. Two loci were detected in 1174 SLE cases and the loci were, an intergenic SNP between FCH and double SH3 domains 2 (FCHSD2) and purinergic receptor P2Y2 (P2RY2), and autophagy related 16 like 2 (ATG16L2). A locus that was detected as suggestive, huntingtin interacting protein 1 (HIP1) is replicated in this study. None of these loci has been as yet detected in Europeans, but in Koreans, several European loci were confirmed once more. These were STAT1-STAT4, TNFSF4, TNFAIP3, IKAROS family zinc finger 1 (IKZF1), HIP1, IRF5, BLK, WDFY family member 4 (WDFY4), ETS proto-oncogene 1 (ETS1) and interleukin 1 receptor associated kinase 1 (IRAK1)-methyl-CpG binding protein 2 (MECP2) [196]. And GWAS was conducted in 4,478 SLE cohort from six East Asian conturies, general transcription factor II-I repeat domain-containing protein 1-general transcription factor 2-I (GTF2IRD1-GTF2I) being the most significant locus among ten new loci [197].
Recently, genetic risk factors have been identified as follows. NADPH oxidase-encoding Ncf1 gene SNP induced production of ROS, so increases the risk of developing SLE [198]. Mutation in the tartrate-resistant acid phosphatase (TRAP)-encoding ACP5 gene result in the expression of IFN-stimulated genes and the production of IL6 and TNF. And an excess of heterozygous ACP5 missense variants was observed in SLE [199]. Transcription factor Blimp1-encoding Prdm1 deficiency in DC led to modulated antigen presentation and the TfH cell repertoire to contribute to autoimmunity [200].
Carriers of HLA-DR4 and DR11 were protected against LN [183]. Conversely HLA-DR3 and DR15 conferred an increased risk of LN. In another approach, a meta-analysis of three GWAS was done to identify risk alleles for LN in patients already known to have SLE [201]. Here the most significant associations for LN mapped to the PDGF receptor A gene and the gene for the sodium-dependent glucose cotransporter solute carrier family 5 member 11 (SLC5A11). In LN, PDGF may mediate kidney cell proliferation, matrix accumulation, and intrarenal inflammation. Several SLC genes have been associated with chronic kidney disease (CKD) [202]. Variants in SLC5A11 may have a role in proximal tubule inositol reabsorption and mediate a decrease in serum and an increase in urine myoinositol [202]. Additionally, SLC5A11 may mediate apoptosis through the programmed cell death and TNF-pathways [203]. HLA loci were less strongly associated with LN in this analysis. Other risk genes include TNFAIP3 interacting protein 1 (ABIN1), TNFSF4, STAT4, ITGAM, kallikreins and FcγRIIIa low-binding alleles [185186204205206207208]. These results suggest a link between inflammation and LN as well as a contribution from pathways that regulate the renal response to inflammation and injury. However the relative risk associated with most of these genetic variants is low.
Epigenetic processes include DNA methylation, post-translational histone modifications were identified in SLE. T cells from patients with active SLE have global DNA hypomethylation [209], especially those from patients with LN [210]. And IFN-stimulated genes (ISGs) were specifically hypomethylated in patients with SLE [210]. Naive CD4+ T cells become primed for Th2, Th17, and TfH cell responses through the activity of the chromatin-modifying enzyme histone–lysine N methyl transferase EZH2 during a flare [211]. Post-translational histone modifications were shown to be aberrant in T cells from patients with SLE. These aberrations were corrected by treatment with mycophenolate mofetil [212]. And histone H4 acetylation was shown to be globally increased in monocytes from patients with SLE [213]. Changes in microRNA (miRNA) expression have been identified in peripheral blood mononuclear cells and renal tissue from patients with SLE [214215216]. MiRNAs identified in patients with SLE seem to affect pathways that affect TLR signaling and expression of ISGs [217218].
Infection has been associated with the occurrence of SLE (Figure 4). Epstein–Barr virus (EBV) and cytomegalovirus are considered to be SLE triggers [219], whereas Helicobacter pylori [220], hepatitis B virus [221], and parasite infections are thought to be protective [222]. Viral infections induce IFNα release, which triggers antiviral immunity as well as lupus disease activity [223]. In addition, EBV activates B cells and contains amino acid sequences similar to those of Ro antigen, resulting in molecular mimicry [224].
The following additional data support the role of microorganisms in SLE. Lipopolysaccharide (LPS) is a component of the cell wall of Gram-negative bacteria that can activate TLR4. Serum levels of LPS are increased in patients with SLE [225] and biomarkers of LPS engagement by TLR4, such as shedding of CD14, correlate with disease activity [226]. Bacterial biofilms represent another mechanism by which microorganisms interact with the immune system. Many biofilms contain amyloid-DNA complex, so greatly increased the production of autoantibodies in lupus-prone mice [227]. Immune stimulation by bacterial DNA results from characteristic sequence motifs that center on an unmethylated cytosine and guanine (CpG) dinucleotide. Bacterial DNA which contain CpG motif more frequently than mammalian DNA presents a structural motif that represents a pathogen associated molecular pattern that can signal a pattern recognition receptor to trigger innate immunity via TLR9 [228]. The pDCs, which responds to TLR9, is a main source of type 1 IFN and therefore has been implicated in lupus pathogenesis [229230]. The microbiome which is the collection of bacteria, viruses, and fungi that coexist on and in the human body is related to SLE. In women with SLE, the ratio of Firmicutesto Bacteroidetes was seen lower than in healthy individuals, even during times of remission [231]. The mechanism of the effect is not fully understood, but certain gut bacteria foster the development of Treg cells [232233].
Furthermore, bacterial products stimulate intrarenal immune cells and renal cells, which can trigger a transient aggravation of proteinuria and kidney damage. Bacterial lipopeptide and LPS aggravate glomerulonephritis and potently induces severe albuminuria in MRL (lpr/lpr) mice [234].
Ultraviolet (UV) light have long been recognized as contributors to SLE (Figure 4) [235]. UV drives apoptosis, providing an immunologic stimulus and increase in the load of dead cells by causing keratinocyte death [236]. And UV decreases DNA methylation level of CD4+ T cells in SLE patients [237238]. UV exposure can induce the secretion of IL-1 and TNFα in keratinocytes, mast cells and Langerhans cells, and can recruit and activate DCs, T cells and pDCs to release IFNα, further releasing chemotactic factors [239].
Drug induced SLE involves inhibition of methyl-transferases, a process that enhances the unmasking of endogenous nucleic acids and the activation of TLR7 and TLR9 [240241]. And UV light converts propranolol into a proinflammatory aryl hydrocarbon receptor ligand, possibly explaining its association with lupus-like disease [242].
Besides, silica exposure from a variety of industrial occupations and smoking is associated with an increased risk of SLE. A longer duration of exposure to silica dust is associated with greater risks [243]. And current smoking and >10 pack-years of smoking with anti-dsDNA positive SLE was observed [244]. And Vitamin D deficiency and zero minor allele of CYP24A1 significantly increased the risk of transitioning to SLE [245]. The alcohol consumption, on the other hand, decrease risk of SLE in large prospective Nurses' Health cohorts (Figure 4) [246].
SLE has a female preponderance of 10:1. The administration of estrogen to postmenopausal women doubles their risk of developing the disease and the inhibition of estradiol in patients with SLE with tamoxifen had modest beneficial effect on the disease [247]. Estrogen enhances immune responses through diverse mechanisms (Figure 1).
The prepubertal ovariectomy of female NZB x NZW F1 (B/W) mouse model for SLE was shown to reduce autoantibody levels, but had no effect on mortality. Conversely, orchiectomy of B/W male mice exhibited accelerated disease onset and shortened life span compared with intact male B/W mice [248]. Treatment with 17 β-estradiol accelerated disease in both female and male B/W mice, while disease was ameliorated in B/W mice treated with testosterone [249]. Treatment of R4A BALB/c mice with 17 β-estradiol resulted in a significant rise in anti-dsDNA antibodies, an increase in the number of anti DNA-secreting B cells [250]. and induced significant aberrations in the selection of autoreactive B cells into the mature B cell pool [251]. In addition, estrogen preferentially increased the proportion of marginal zone B cells [251], which participates in T independent immune responses. Estrogen treatment blocked BCR mediated apoptosis, which is essential for the elimination of autoreactive B cells [251252]. The antiapoptotic Bcl-2 protein, which is a known estrogen target [36], was increased in estrogen treated mice [250253]. An increase in Bcl-2 may enhance the survival of B cells that would normally be eliminated by tolerogenic signals [254].
In human SLE studies, the concentration of estradiol and prolactin in lupus patients was increased [255]. However, the level of these hormones is not related to the severity of SLE, and the concentration is within the physiological range. Females with SLE have even lower androgen levels than their normal female counterparts. The oxidation of androgens in SLE is increased when compared to males with the disease [256257]. The expression of intracellular ER-beta which is anti-inflammatory was reduced in T cells from SLE patients with SLEDAI-2K scores > 6 as compared to those with scores <6 or healthy controls. No difference was found for ER alpha differences [258].
Treatment of R4A BALB/c mice with 17 β-estradiol induced resulted rising in anti DNA antibody titers and immune complexes depositions in the kidneys [250]. And treatment of R4A BALB/c mice with tamoxifen prevented the estrogen-induced production of anti-DNA antibodies and immune complex deposition in the kidney [259].
SLE is a systemic autoimmune disease with multi-organ inflammation. Accelerated cell death and formation of NETs activate pDC TLR 9 and induce IFN αcontributing to the development of SLE, especially LN. Many cytokines such as IFN, TNF, IL-4, IL-6, IL-10, and activated complement system enhanced the promotion of autoantibody production and inflammation. Loss of tolerance and altered B-cell and T cell differentiation in SLE might present from birth or acquired as part of the disease process of LN. Over 100 genes have been associated with susceptibility to SLE such as HLA, FcγRIIA and FcγRIIIB, ITGAM, deficiency of C2,C4, and C1q. Environmental factors such as infection (EBV, CMV, microbiome), UV light,smoking, some drugs, silica, Vitamin Ddeficiency increased the risk of transitioning to SLE. We are still far away from really knowing the pathogenesis of LN. However, the development of target therapies and an eventual cure will come in our lifetime.
Figures and Tables
 | Figure 1Overall pathogenic mechanism of lupus nephritis. HLA: human leukocyte antigen, SLC5A11: solute carrier family 5 member 11, PDGF: platelet-derived growth factor, TNFSF4: tumor necrosis factor ligand superfamily, member 4, ITGAM: integrin alpha M, STAT: signal transducer and activator of transcription, UV: ultraviolet, RNA: ribonucleic acid, DNA: deoxyribonucleic acid, DNase: deoxyribonuclease, pDC: plasmacytoid dendritic cells, TLR: toll-like receptor, STING: stimulator of interferon gene, IFN: interferon, TNF: tumor necrosis factor, IL: interleukin, BAFF: B cell-activating factor, CCL2: chemokine ligand 2, FGF: fibroblast growth factor, Th: helper T cells, Treg: regulatory T cells, TfH: follicular helper T cells, dsDNA: double strand DNA. |
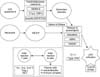 | Figure 2Potential role of aberrant cell death in the development of systemic lupus erythematous. CRP: C-reactive protein, PTX: pentraxin-related protein, DNase: deoxyribonuclease, NET: neutrophil extracellular trap, DNA: deoxyribonucleic acid, RNA: ribonucleic acid, AMP: adenosine monophosphate, HMGB1: high-mobility group box 1 protein, pDC: plasmacytoid dendritic cells, TLR: toll-like receptor, STING: stimulator of interferon gene, IFN: interferon, Th: helper T cells, Treg: regulatory T cells, TfH: follicular helper T cells. |
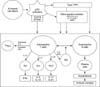 | Figure 3Innate and acquired immunity processes contribute to systemic lupus erythematous. pDC: plasmacytoid dendritic cells, TLR: toll-like receptor, STING: stimulator of interferon gene, IFN: interferon, TNF: tumor necrosis factor, IL: interleukin, BAFF: B cell-activating factor, Treg: regulatory T cells, Th: helper T cells, TfH: follicular helper T cells. |
 | Figure 4Associated genes and environmental factors with the pathogenesis of systemic lupus erythematous. CRP: C-reactive protein, ATG5: α-glucoside transporter 5, TREX1: three prime repair exonuclease 1, UV: ultraviolet, HLA: human leukocyte antigen, FCGR: Fcγ receptor, IRAK: interleukin 1 receptor associated kinase, IRF: IFN regulatory factor, STAT: signal transducer and activator of transcription, ITGAM: integrin α M, TNFAIP: TNF α-induced protein, IFI27: interferon α-inducible protein 27, IFI44L: interferon-induced protein 44-like, LY6E: lymphocyte antigen 6 complex, locus E, IFITM1: interferon induced transmembrane protein 1, BANK1: B cell scaffold protein with ankyrin repeats 1, BLK: B lymphoid tyrosine kinase, ETS1: ETS proto-oncogene 1, PTPN22: protein tyrosine phosphatase, nonreceptor type 22, TNFSF: tumor necrosis factor ligand superfamily, member, IKZF1: IKAROS family zinc finger 1, SLC5A11: solute carrier family 5 member 11, PDGFR: platelet-derived growth factor receptor, NCF1: neutrophil cytosolic factor 1, ACP5: acid phosphatase 5, PRDM1: PR domain zinc finger protein 1. |
ACKNOWLEDGMENTS
We would like to thank Aldus Manutius and Ludovico Arrighi who created the Italic type.
References
1. Shlomchik MJ, Craft JE, Mamula MJ. From T to B and back again: positive feedback in systemic autoimmune disease. Nat Rev Immunol. 2001; 1:147–153.

2. Ortega LM, Schultz DR, Lenz O, Pardo V, Contreras GN. Review: Lupus nephritis: pathologic features, epidemiology and a guide to therapeutic decisions. Lupus. 2010; 19:557–574.

3. Shim JS, Sung YK, Joo YB, Lee HS, Bae SC. Prevalence and incidence of systemic lupus erythematosus in South Korea. Rheumatol Int. 2014; 34:909–917.

4. Hsu CY, Chiu WC, Yang TS, Chen CJ, Chen YC, Lai HM, et al. Age- and gender-related long-term renal outcome in patients with lupus nephritis. Lupus. 2011; 20:1135–1141.

5. Merrell M, Shulman LE. Determination of prognosis in chronic disease, illustrated by systemic lupus erythematosus. J Chronic Dis. 1955; 1:12–32.

6. Pons-Estel GJ, Alarcón GS, Scofield L, Reinlib L, Cooper GS. Understanding the epidemiology and progression of systemic lupus erythematosus. Semin Arthritis Rheum. 2010; 39:257–268.

7. Maroz N, Segal MS. Lupus nephritis and end-stage kidney disease. Am J Med Sci. 2013; 346:319–323.

8. Anaya JM, Cañas C, Mantilla RD, Pineda-Tamayo R, Tobón GJ, Herrera-Diaz C, et al. Lupus nephritis in Colombians: contrasts and comparisons with other populations. Clin Rev Allergy Immunol. 2011; 40:199–207.

9. Yap DY, Tang CS, Ma MK, Lam MF, Chan TM. Survival analysis and causes of mortality in patients with lupus nephritis. Nephrol Dial Transplant. 2012; 27:3248–3254.

10. Lerang K, Gilboe IM, Steinar Thelle D, Gran JT. Mortality and years of potential life loss in systemic lupus erythematosus: a population-based cohort study. Lupus. 2014; 23:1546–1552.

11. Bernatsky S, Boivin JF, Joseph L, Manzi S, Ginzler E, Gladman DD, et al. Mortality in systemic lupus erythematosus. Arthritis Rheum. 2006; 54:2550–2557.

12. Faurschou M, Dreyer L, Kamper AL, Starklint H, Jacobsen S. Long-term mortality and renal outcome in a cohort of 100 patients with lupus nephritis. Arthritis Care Res (Hoboken). 2010; 62:873–880.

13. Pieterse E, van der. Breaking immunological tolerance in systemic lupus erythematosus. Front Immunol. 2014; 5:164.

14. Lynch DH, Watson ML, Alderson MR, Baum PR, Miller RE, Tough T, et al. The mouse Fas-ligand gene is mutated in gld mice and is part of a TNF family gene cluster. Immunity. 1994; 1:131–136.

15. Takahashi T, Tanaka M, Brannan CI, Jenkins NA, Copeland NG, Suda T, et al. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994; 76:969–976.

16. Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992; 356:314–317.

17. Fisher GH, Rosenberg FJ, Straus SE, Dale JK, Middleton LA, Lin AY, et al. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell. 1995; 81:935–946.

18. Rieux-Laucat F, Le Deist F, Hivroz C, Roberts IA, Debatin KM, Fischer A, et al. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science. 1995; 268:1347–1349.

19. Gaipl US, Voll RE, Sheriff A, Franz S, Kalden JR, Herrmann M. Impaired clearance of dying cells in systemic lupus erythematosus. Autoimmun Rev. 2005; 4:189–194.

20. Muñoz LE, Lauber K, Schiller M, Manfredi AA, Herrmann M. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat Rev Rheumatol. 2010; 6:280–289.

21. van Bavel CC, Dieker J, Muller S, Briand JP, Monestier M, Berden JH, et al. Apoptosis-associated acetylation on histone H2B is an epitope for lupus autoantibodies. Mol Immunol. 2009; 47:511–516.

22. Dieker JW, Fransen JH, van Bavel CC, Briand JP, Jacobs CW, Muller S, et al. Apoptosis-induced acetylation of histones is pathogenic in systemic lupus erythematosus. Arthritis Rheum. 2007; 56:1921–1933.

23. Plaué S, Muller S, van Regenmortel MH. A branched, synthetic octapeptide of ubiquitinated histone H2A as target of autoantibodies. J Exp Med. 1989; 169:1607–1617.

24. Dieker J, Tel J, Pieterse E, Thielen A, Rother N, Bakker M, et al. Circulating apoptotic microparticles in systemic lupus erythematosus patients drive the activation of dendritic cell subsets and prime neutrophils for NETosis. Arthritis Rheumatol. 2016; 68:462–472.

25. Dieker JW, van der Vlag J, Berden JH. Deranged removal of apoptotic cells: its role in the genesis of lupus. Nephrol Dial Transplant. 2004; 19:282–285.

26. Soto HM, Parra G, Rodríguez-Itrube B. Circulating levels of cytokines in poststreptococcal glomerulonephritis. Clin Nephrol. 1997; 47:6–12.
27. Makino H, Sugiyama H, Yamasaki Y, Maeshima Y, Wada J, Kashihara N. Glomerular cell apoptosis in human lupus nephritis. Virchows Arch. 2003; 443:67–77.
28. Soto H, Mosquera J, Rodríguez-Iturbe B, Henriquez La, Pinto A. Apoptosis in proliferative glomerulonephritis: decreased apoptosis expression in lupus nephritis. Nephrol Dial Transplant. 1997; 12:273–280.

29. Faurschou M, Penkowa M, Andersen CB, Starklint H, Jacobsen S. Renal cell apoptosis in human lupus nephritis: a histological study. Lupus. 2009; 18:994–999.

30. Watanabe M, Hitomi M, van der Wee K, Rothenberg F, Fisher SA, Zucker R, et al. The pros and cons of apoptosis assays for use in the study of cells, tissues, and organs. Microsc Microanal. 2002; 8:375–391.

31. van Bavel CC, Dieker JW, Kroeze Y, Tamboer WP, Voll R, Muller S, et al. Apoptosis-induced histone H3 methylation is targeted by autoantibodies in systemic lupus erythematosus. Ann Rheum Dis. 2011; 70:201–207.

32. Stöckl F, Muller S, Batsford S, Schmiedeke T, Waldherr R, Andrassy K, et al. A role for histones and ubiquitin in lupus nephritis? Clin Nephrol. 1994; 41:10–17.
33. Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010; 191:677–691.

34. Saffarzadeh M, Preissner KT. Fighting against the dark side of neutrophil extracellular traps in disease: manoeuvres for host protection. Curr Opin Hematol. 2013; 20:3–9.
35. Villanueva E, Yalavarthi S, Berthier CC, Hodgin JB, Khandpur R, Lin AM, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol. 2011; 187:538–552.

36. Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med. 2011; 3:73ra20.

37. Knight JS, Subramanian V, O'Dell AA, Yalavarthi S, Zhao W, Smith CK, et al. Peptidylarginine deiminase inhibition disrupts NET formation and protects against kidney, skin and vascular disease in lupus-prone MRL/lpr mice. Ann Rheum Dis. 2015; 74:2199–2206.

38. Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med. 2011; 3:73ra19.

39. Napirei M, Karsunky H, Zevnik B, Stephan H, Mannherz HG, Möröy T. Features of systemic lupus erythematosus in Dnase1-deficient mice. Nat Genet. 2000; 25:177–181.

40. Skiljevic D, Jeremic I, Nikolic M, Andrejevic S, Sefik-Bukilica M, Stojimirovic B, et al. Serum DNase I activity in systemic lupus erythematosus: correlation with immunoserological markers, the disease activity and organ involvement. Clin Chem Lab Med. 2013; 51:1083–1091.

41. Hakkim A, Fürnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A. 2010; 107:9813–9818.

42. Leffler J, Martin M, Gullstrand B, Tydén H, Lood C, Truedsson L, et al. Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease. J Immunol. 2012; 188:3522–3531.

43. Bodaño A, González A, Ferreiros-Vidal I, Balada E, Ordi J, Carreira P, et al. Association of a non-synonymous single-nucleotide polymorphism of DNASEI with SLE susceptibility. Rheumatology (Oxford). 2006; 45:819–823.
44. Theofilopoulos AN, Kono DH, Beutler B, Baccala R. Intracellular nucleic acid sensors and autoimmunity. J Interferon Cytokine Res. 2011; 31:867–886.

45. Celhar T, Magalhães R, Fairhurst AM. TLR7 and TLR9 in SLE: when sensing self goes wrong. Immunol Res. 2012; 53:58–77.

46. Komatsuda A, Wakui H, Iwamoto K, Ozawa M, Togashi M, Masai R, et al. Up-regulated expression of Toll-like receptors mRNAs in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Clin Exp Immunol. 2008; 152:482–487.

47. Lyn-Cook BD, Xie C, Oates J, Treadwell E, Word B, Hammons G, et al. Increased expression of Toll-like receptors (TLRs) 7 and 9 and other cytokines in systemic lupus erythematosus (SLE) patients: ethnic differences and potential new targets for therapeutic drugs. Mol Immunol. 2014; 61:38–43.

48. Chauhan SK, Singh VV, Rai R, Rai M, Rai G. Distinct autoantibody profiles in systemic lupus erythematosus patients are selectively associated with TLR7 and TLR9 upregulation. J Clin Immunol. 2013; 33:954–964.

49. Papadimitraki ED, Choulaki C, Koutala E, Bertsias G, Tsatsanis C, Gergianaki I, et al. Expansion of toll-like receptor 9-expressing B cells in active systemic lupus erythematosus: implications for the induction and maintenance of the autoimmune process. Arthritis Rheum. 2006; 54:3601–3611.

50. Christensen SR, Kashgarian M, Alexopoulou L, Flavell RA, Akira S, Shlomchik MJ. Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J Exp Med. 2005; 202:321–331.

51. Flür K, Allam R, Zecher D, Kulkarni OP, Lichtnekert J, Schwarz M, et al. Viral RNA induces type I interferon-dependent cytokine release and cell death in mesangial cells via melanoma-differentiation-associated gene-5: Implications for viral infection-associated glomerulonephritis. Am J Pathol. 2009; 175:2014–2022.
52. Allam R, Lichtnekert J, Moll AG, Taubitz A, Vielhauer V, Anders HJ. Viral RNA and DNA trigger common antiviral responses in mesangial cells. J Am Soc Nephrol. 2009; 20:1986–1996.

53. Hägele H, Allam R, Pawar RD, Anders HJ. Double-stranded RNA activates type I interferon secretion in glomerular endothelial cells via retinoic acid-inducible gene (RIG)-1. Nephrol Dial Transplant. 2009; 24:3312–3318.
54. Hägele H, Allam R, Pawar RD, Reichel CA, Krombach F, Anders HJ. Double-stranded DNA activates glomerular endothelial cells and enhances albumin permeability via a toll-like receptor-independent cytosolic DNA recognition pathway. Am J Pathol. 2009; 175:1896–1904.

55. Fairhurst AM, Xie C, Fu Y, Wang A, Boudreaux C, Zhou XJ, et al. Type I interferons produced by resident renal cells may promote end-organ disease in autoantibody-mediated glomerulonephritis. J Immunol. 2009; 183:6831–6838.

56. Anders HJ, Lichtnekert J, Allam R. Interferon-alpha and -beta in kidney inflammation. Kidney Int. 2010; 77:848–854.
57. Triantafyllopoulou A, Franzke CW, Seshan SV, Perino G, Kalliolias GD, Ramanujam M, et al. Proliferative lesions and metalloproteinase activity in murine lupus nephritis mediated by type I interferons and macrophages. Proc Natl Acad Sci U S A. 2010; 107:3012–3017.

58. Anders HJ, Vielhauer V, Eis V, Linde Y, Kretzler M, Perez de Lema G, et al. Activation of toll-like receptor-9 induces progression of renal disease in MRL-Fas(lpr) mice. FASEB J. 2004; 18:534–536.

59. Savarese E, Steinberg C, Pawar RD, Reindl W, Akira S, Anders HJ, et al. Requirement of Toll-like receptor 7 for pristane-induced production of autoantibodies and development of murine lupus nephritis. Arthritis Rheum. 2008; 58:1107–1115.

60. Pawar RD, Patole PS, Zecher D, Segerer S, Kretzler M, Schlöndorff D, et al. Toll-like receptor-7 modulates immune complex glomerulonephritis. J Am Soc Nephrol. 2006; 17:141–149.

61. Pawar RD, Ramanjaneyulu A, Kulkarni OP, Lech M, Segerer S, Anders HJ. Inhibition of Toll-like receptor-7 (TLR-7) or TLR-7 plus TLR-9 attenuates glomerulonephritis and lung injury in experimental lupus. J Am Soc Nephrol. 2007; 18:1721–1731.

62. Subramanian S, Tus K, Li QZ, Wang A, Tian XH, Zhou J, et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc Natl Acad Sci U S A. 2006; 103:9970–9975.

63. Ramirez-Ortiz ZG, Prasad A, Griffith JW, Pendergraft WF 3rd, Cowley GS, Root DE, et al. The receptor TREML4 amplifies TLR7-mediated signaling during antiviral responses and autoimmunity. Nat Immunol. 2015; 16:495–504.

64. Patole PS, Gröne HJ, Segerer S, Ciubar R, Belemezova E, Henger A, et al. Viral double-stranded RNA aggravates lupus nephritis through Toll-like receptor 3 on glomerular mesangial cells and antigen-presenting cells. J Am Soc Nephrol. 2005; 16:1326–1338.

65. Imaizumi T, Aizawa T, Segawa C, Shimada M, Tsuruga K, Kawaguchi S, et al. Toll-like receptor 3 signaling contributes to the expression of a neutrophil chemoattractant, CXCL1 in human mesangial cells. Clin Exp Nephrol. 2015; 19:761–770.

66. Tian J, Ma Y, Li J, Cen H, Wang DG, Feng CC, et al. The TLR7 7926A>G polymorphism is associated with susceptibility to systemic lupus erythematosus. Mol Med Rep. 2012; 6:105–110.
67. Ramachandran R, Sharma V, Rathi M, Yadav AK, Sharma A, Kohli HS, et al. Association between -1486 T>C and +1174 G>A single nucleotide polymorphisms in TLR9 gene and severity of lupus nephritis. Indian J Nephrol. 2012; 22:125–129.
68. Munroe ME, Lu R, Zhao YD, Fife DA, Robertson JM, Guthridge JM, et al. Altered type II interferon precedes autoantibody accrual and elevated type I interferon activity prior to systemic lupus erythematosus classification. Ann Rheum Dis. 2016; 75:2014–2021.

69. Weckerle CE, Mangale D, Franek BS, Kelly JA, Kumabe M, James JA, et al. Large-scale analysis of tumor necrosis factor α levels in systemic lupus erythematosus. Arthritis Rheum. 2012; 64:2947–2952.
70. Yarilina A, Park-Min KH, Antoniv T, Hu X, Ivashkiv LB. TNF activates an IRF1-dependent autocrine loop leading to sustained expression of chemokines and STAT1-dependent type I interferon-response genes. Nat Immunol. 2008; 9:378–387.

71. Flint SM, Jovanovic V, Teo BW, Mak A, Thumboo J, McKinney EF, et al. Leucocyte subset-specific type 1 interferon signatures in SLE and other immune-mediated diseases. RMD Open. 2016; 2:e000183.

72. Liu Z, Bethunaickan R, Huang W, Lodhi U, Solano I, Madaio MP, et al. Interferon-α accelerates murine systemic lupus erythematosus in a T cell-dependent manner. Arthritis Rheum. 2011; 63:219–229.

73. Fairhurst AM, Mathian A, Connolly JE, Wang A, Gray HF, George TA, et al. Systemic IFN-alpha drives kidney nephritis in B6.Sle123 mice. Eur J Immunol. 2008; 38:1948–1960.
74. Solomou EE, Juang YT, Gourley MF, Kammer GM, Tsokos GC. Molecular basis of deficient IL-2 production in T cells from patients with systemic lupus erythematosus. J Immunol. 2001; 166:4216–4222.

75. Li D, Guo B, Wu H, Tan L, Chang C, Lu Q. Interleukin-17 in systemic lupus erythematosus: a comprehensive review. Autoimmunity. 2015; 48:353–361.

76. Kyttaris VC, Kampagianni O, Tsokos GC. Treatment with anti-interleukin 23 antibody ameliorates disease in lupus-prone mice. Biomed Res Int. 2013; 2013:861028.

77. Pers JO, Daridon C, Devauchelle V, Jousse S, Saraux A, Jamin C, et al. BAFF overexpression is associated with autoantibody production in autoimmune diseases. Ann N Y Acad Sci. 2005; 1050:34–39.

78. Stohl W, Xu D, Kim KS, Koss MN, Jorgensen TN, Deocharan B, et al. BAFF overexpression and accelerated glomerular disease in mice with an incomplete genetic predisposition to systemic lupus erythematosus. Arthritis Rheum. 2005; 52:2080–2091.

79. Liu Z, Davidson A. BAFF and selection of autoreactive B cells. Trends Immunol. 2011; 32:388–394.

80. Bethunaickan R, Berthier CC, Zhang W, Eksi R, Li HD, Guan Y, et al. Identification of stage-specific genes associated with lupus nephritis and response to remission induction in (NZB × NZW)F1 and NZM2410 mice. Arthritis Rheumatol. 2014; 66:2246–2258.

81. Pérez de Lema G, Maier H, Nieto E, Vielhauer V, Luckow B, Mampaso F, et al. Chemokine expression precedes inflammatory cell infiltration and chemokine receptor and cytokine expression during the initiation of murine lupus nephritis. J Am Soc Nephrol. 2001; 12:1369–1382.
82. Liu J, Karypis G, Hippen KL, Vegoe AL, Ruiz P, Gilkeson GS, et al. Genomic view of systemic autoimmunity in MRLlpr mice. Genes Immun. 2006; 7:156–168.

83. Berthier CC, Bethunaickan R, Gonzalez-Rivera T, Nair V, Ramanujam M, Zhang W, et al. Cross-species transcriptional network analysis defines shared inflammatory responses in murine and human lupus nephritis. J Immunol. 2012; 189:988–1001.

84. Migliorini A, Angelotti ML, Mulay SR, Kulkarni OO, Demleitner J, Dietrich A, et al. The antiviral cytokines IFN-α and IFN-β modulate parietal epithelial cells and promote podocyte loss: implications for IFN toxicity, viral glomerulonephritis, and glomerular regeneration. Am J Pathol. 2013; 183:431–440.
85. Gurkan S, Cabinian A, Lopez V, Bhaumik M, Chang JM, Rabson AB, et al. Inhibition of type I interferon signalling prevents TLR ligand-mediated proteinuria. J Pathol. 2013; 231:248–256.

86. Tesch GH, Maifert S, Schwarting A, Rollins BJ, Kelley VR. Monocyte chemoattractant protein 1-dependent leukocytic infiltrates are responsible for autoimmune disease in MRL-Fas(lpr) mice. J Exp Med. 1999; 190:1813–1824.

87. Bethunaickan R, Sahu R, Liu Z, Tang YT, Huang W, Edegbe O, et al. Anti-tumor necrosis factor α treatment of interferon-α-induced murine lupus nephritis reduces the renal macrophage response but does not alter glomerular immune complex formation. Arthritis Rheum. 2012; 64:3399–3408.

88. Tucci M, Stucci S, Strippoli S, Silvestris F. Cytokine overproduction, T-cell activation, and defective T-regulatory functions promote nephritis in systemic lupus erythematosus. J Biomed Biotechnol. 2010; 2010:457146.

89. Boswell JM, Yui MA, Burt DW, Kelley VE. Increased tumor necrosis factor and IL-1 beta gene expression in the kidneys of mice with lupus nephritis. J Immunol. 1988; 141:3050–3054.
90. Herrera-Esparza R, Barbosa-Cisneros O, Villalobos-Hurtado R, Avalos-Díaz E. Renal expression of IL-6 and TNFalpha genes in lupus nephritis. Lupus. 1998; 7:154–158.
91. Zhao J, Wang H, Dai C, Wang H, Zhang H, Huang Y, et al. P2X7 blockade attenuates murine lupus nephritis by inhibiting activation of the NLRP3/ASC/caspase 1 pathway. Arthritis Rheum. 2013; 65:3176–3185.
92. Kahlenberg JM, Kaplan MJ. The inflammasome and lupus: another innate immune mechanism contributing to disease pathogenesis? Curr Opin Rheumatol. 2014; 26:475–481.
93. Zhao J, Wang H, Huang Y, Zhang H, Wang S, Gaskin F, et al. Lupus nephritis: glycogen synthase kinase 3β promotion of renal damage through activation of the NLRP3 inflammasome in lupus-prone mice. Arthritis Rheumatol. 2015; 67:1036–1044.

94. Amarilyo G, Lourenço EV, Shi FD, La Cava A. IL-17 promotes murine lupus. J Immunol. 2014; 193:540–543.

95. Schmidt T, Paust HJ, Krebs CF, Turner JE, Kaffke A, Bennstein SB, et al. Function of the Th17/interleukin-17A immune response in murine lupus nephritis. Arthritis Rheumatol. 2015; 67:475–487.

96. Liarski VM, Kaverina N, Chang A, Brandt D, Yanez D, Talasnik L, et al. Cell distance mapping identifies functional T follicular helper cells in inflamed human renal tissue. Sci Transl Med. 2014; 6:230ra46.

97. Lee SK, Silva DG, Martin JL, Pratama A, Hu X, Chang PP, et al. Interferon-γ excess leads to pathogenic accumulation of follicular helper T cells and germinal centers. Immunity. 2012; 37:880–892.

98. Miyake K, Nakashima H, Akahoshi M, Inoue Y, Nagano S, Tanaka Y, et al. Genetically determined interferon-gamma production influences the histological phenotype of lupus nephritis. Rheumatology (Oxford). 2002; 41:518–524.

99. Namjou B, Sestak AL, Armstrong DL, Zidovetzki R, Kelly JA, Jacob N, et al. High-density genotyping of STAT4 reveals multiple haplotypic associations with systemic lupus erythematosus in different racial groups. Arthritis Rheum. 2009; 60:1085–1095.

100. Pickering MC, Botto M, Taylor PR, Lachmann PJ, Walport MJ. Systemic lupus erythematosus, complement deficiency, and apoptosis. Adv Immunol. 2000; 76:227–324.

101. Barilla-LaBarca ML, Atkinson JP. Rheumatic syndromes associated with complement deficiency. Curr Opin Rheumatol. 2003; 15:55–60.

102. Walport MJ, Davies KA, Botto M. C1q and systemic lupus erythematosus. Immunobiology. 1998; 199:265–285.

103. Kallel-Sellami M, Baili-Klila L, Zerzeri Y, Laadhar L, Blouin J, Abdelmalek R, et al. Hereditary complement deficiency and lupus: report of four Tunisian cases. Ann N Y Acad Sci. 2007; 1108:197–202.

104. Mayilyan KR. Complement genetics, deficiencies, and disease associations. Protein Cell. 2012; 3:487–496.

105. Laich A, Sim RB. Cross-talk between the human complement classical and alternative pathways: evidence for a C4bBb 'hybrid' C3 convertase. Mol Immunol. 2001; 38:105.
106. Jönsson G, Sjöholm AG, Truedsson L, Bengtsson AA, Braconier JH, Sturfelt G. Rheumatological manifestations, organ damage and autoimmunity in hereditary C2 deficiency. Rheumatology (Oxford). 2007; 46:1133–1139.

107. Stegert M, Bock M, Trendelenburg M. Clinical presentation of human C1q deficiency: How much of a lupus? Mol Immunol. 2015; 67:3–11.

108. Beurskens FJ, van Schaarenburg RA, Trouw LA. C1q, antibodies and anti-C1q autoantibodies. Mol Immunol. 2015; 68:6–13.

109. Sinico RA, Rimoldi L, Radice A, Bianchi L, Gallelli B, Moroni G. Anti-C1q autoantibodies in lupus nephritis. Ann N Y Acad Sci. 2009; 1173:47–51.

110. Birmingham DJ, Bitter JE, Ndukwe EG, Dials S, Gullo TR, Conroy S, et al. Relationship of circulating anti-C3b and anti-C1q IgG to lupus nephritis and its flare. Clin J Am Soc Nephrol. 2016; 11:47–53.

111. Gargiulo Mde L, Gómez G, Khoury M, Collado MV, Suárez L, Álvarez C, et al. Association between the presence of anti-C1q antibodies and active nephritis in patients with systemic lupus erythematosus. Medicina (B Aires). 2015; 75:23–28.
112. Leffler J, Bengtsson AA, Blom AM. The complement system in systemic lupus erythematosus: an update. Ann Rheum Dis. 2014; 73:1601–1606.

113. Jönsen A, Gunnarsson I, Gullstrand B, Svenungsson E, Bengtsson AA, Nived O, et al. Association between SLE nephritis and polymorphic variants of the CRP and FcgammaRIIIa genes. Rheumatology (Oxford). 2007; 46:1417–1421.
114. Seelen MA, van der Bijl EA, Trouw LA, Zuiverloon TC, Munoz JR, Fallaux-van den Houten FC, et al. A role for mannose-binding lectin dysfunction in generation of autoantibodies in systemic lupus erythematosus. Rheumatology (Oxford). 2005; 44:111–119.

115. Dörner T, Jacobi AM, Lee J, Lipsky PE. Abnormalities of B cell subsets in patients with systemic lupus erythematosus. J Immunol Methods. 2011; 363:187–197.

116. Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003; 301:1374–1377.

117. Yurasov S, Tiller T, Tsuiji M, Velinzon K, Pascual V, Wardemann H, et al. Persistent expression of autoantibodies in SLE patients in remission. J Exp Med. 2006; 203:2255–2261.

118. Guerrier T, Youinou P, Pers JO, Jamin C. TLR9 drives the development of transitional B cells towards the marginal zone pathway and promotes autoimmunity. J Autoimmun. 2012; 39:173–179.

119. Thien M, Phan TG, Gardam S, Amesbury M, Basten A, Mackay F, et al. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004; 20:785–798.

120. Lesley R, Xu Y, Kalled SL, Hess DM, Schwab SR, Shu HB, et al. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity. 2004; 20:441–453.

121. Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999; 190:1697–1710.

122. Gao N, Dresel J, Eckstein V, Gellert R, Störch H, Venigalla RK, et al. Impaired suppressive capacity of activation-induced regulatory B cells in systemic lupus erythematosus. Arthritis Rheumatol. 2014; 66:2849–2861.

124. Bohnhorst JØ, Bjørgan MB, Thoen JE, Natvig JB, Thompson KM. Bm1-Bm5 classification of peripheral blood B cells reveals circulating germinal center founder cells in healthy individuals and disturbance in the B cell subpopulations in patients with primary Sjögren's syndrome. J Immunol. 2001; 167:3610–3618.

125. Harada Y, Kawano MM, Huang N, Mahmoud MS, Lisukov IA, Mihara K, et al. Identification of early plasma cells in peripheral blood and their clinical significance. Br J Haematol. 1996; 92:184–191.

126. Nimmerjahn F, Ravetch JV. Fc-receptors as regulators of immunity. Adv Immunol. 2007; 96:179–204.

127. Ettinger R, Kuchen S, Lipsky PE. Interleukin 21 as a target of intervention in autoimmune disease. Ann Rheum Dis. 2008; 67:Suppl 3. iii83–iii86.

128. Goilav B, Putterman C. The role of Anti-DNA antibodies in the development of lupus nephritis: a complementary, or alternative, viewpoint? Semin Nephrol. 2015; 35:439–443.

129. Sabbaga J, Pankewycz OG, Lufft V, Schwartz RS, Madaio MP. Cross-reactivity distinguishes serum and nephritogenic anti-DNA antibodies in human lupus from their natural counterparts in normal serum. J Autoimmun. 1990; 3:215–235.

130. Xie C, Liang Z, Chang S, Mohan C. Use of a novel elution regimen reveals the dominance of polyreactive antinuclear autoantibodies in lupus kidneys. Arthritis Rheum. 2003; 48:2343–2352.

131. Kalaaji M, Sturfelt G, Mjelle JE, Nossent H, Rekvig OP. Critical comparative analyses of anti-alpha-actinin and glomerulus-bound antibodies in human and murine lupus nephritis. Arthritis Rheum. 2006; 54:914–926.
132. Mannik M, Merrill CE, Stamps LD, Wener MH. Multiple autoantibodies form the glomerular immune deposits in patients with systemic lupus erythematosus. J Rheumatol. 2003; 30:1495–1504.
133. Seredkina N, Van Der Vlag J, Berden J, Mortensen E, Rekvig OP. Lupus nephritis: enigmas, conflicting models and an emerging concept. Mol Med. 2013; 19:161–169.

134. Fenton K, Fismen S, Hedberg A, Seredkina N, Fenton C, Mortensen ES, et al. Anti-dsDNA antibodies promote initiation, and acquired loss of renal Dnase1 promotes progression of lupus nephritis in autoimmune (NZBxNZW)F1 mice. PLoS One. 2009; 4:e8474.

135. Seredkina N, Rekvig OP. Acquired loss of renal nuclease activity is restricted to DNaseI and is an organ-selective feature in murine lupus nephritis. Am J Pathol. 2011; 179:1120–1128.

136. Rekvig OP. The anti-DNA antibody: origin and impact, dogmas and controversies. Nat Rev Rheumatol. 2015; 11:530–540.

137. Adu D, Dobson J, Williams DG. DNA-anti-DNA circulating complexes in the nephritis of systemic lupus erythematosus. Clin Exp Immunol. 1981; 43:605–614.
138. Fenton KA, Tømmerås B, Marion TN, Rekvig OP. Pure anti-dsDNA mAbs need chromatin structures to promote glomerular mesangial deposits in BALB/c mice. Autoimmunity. 2010; 43:179–188.

139. Ehrenstein MR, Katz DR, Griffiths MH, Papadaki L, Winkler TH, Kalden JR, et al. Human IgG anti-DNA antibodies deposit in kidneys and induce proteinuria in SCID mice. Kidney Int. 1995; 48:705–711.

140. Bruschi M, Sinico RA, Moroni G, Pratesi F, Migliorini P, Galetti M, et al. Glomerular autoimmune multicomponents of human lupus nephritis in vivo: α-enolase and annexin AI. J Am Soc Nephrol. 2014; 25:2483–2498.

141. Chang A, Henderson SG, Brandt D, Liu N, Guttikonda R, Hsieh C, et al. In situ B cell-mediated immune responses and tubulointerstitial inflammation in human lupus nephritis. J Immunol. 2011; 186:1849–1860.

142. Espeli M, Bökers S, Giannico G, Dickinson HA, Bardsley V, Fogo AB, et al. Local renal autoantibody production in lupus nephritis. J Am Soc Nephrol. 2011; 22:296–305.

143. Kinloch AJ, Chang A, Ko K, Henry Dunand CJ, Henderson S, et al. Vimentin is a dominant target of in situ humoral immunity in human lupus tubulointerstitial nephritis. Arthritis Rheumatol. 2014; 66:3359–3370.

144. Rose ML. Role of anti-vimentin antibodies in allograft rejection. Hum Immunol. 2013; 74:1459–1462.

145. Packard TA, Cambier JC. B lymphocyte antigen receptor signaling: initiation, amplification, and regulation. F1000Prime Rep. 2013; 5:40.

146. Manjarrez-Orduño N, Marasco E, Chung SA, Katz MS, Kiridly JF, Simpfendorfer KR, et al. CSK regulatory polymorphism is associated with systemic lupus erythematosus and influences B-cell signaling and activation. Nat Genet. 2012; 44:1227–1230.

147. Lu R, Vidal GS, Kelly JA, Delgado-Vega AM, Howard XK, Macwana SR, et al. Genetic associations of LYN with systemic lupus erythematosus. Genes Immun. 2009; 10:397–403.

148. Patole PS, Zecher D, Pawar RD, Gröne HJ, Schlöndorff D, Anders HJ. G-rich DNA suppresses systemic lupus. J Am Soc Nephrol. 2005; 16:3273–3280.

149. Peters AL, Plenge RM, Graham RR, Altshuler DM, Moser KL, Gaffney PM, et al. A novel polymorphism of the human CD40 receptor with enhanced function. Blood. 2008; 112:1863–1871.

150. Enyedy EJ, Nambiar MP, Liossis SN, Dennis G, Kammer GM, Tsokos GC. Fc epsilon receptor type I gamma chain replaces the deficient T cell receptor zeta chain in T cells of patients with systemic lupus erythematosus. Arthritis Rheum. 2001; 44:1114–1121.
151. Liossis SN, Ding XZ, Dennis GJ, Tsokos GC. Altered pattern of TCR/CD3-mediated protein-tyrosyl phosphorylation in T cells from patients with systemic lupus erythematosus. Deficient expression of the T cell receptor zeta chain. J Clin Invest. 1998; 101:1448–1457.

152. Kyttaris VC, Juang YT, Tenbrock K, Weinstein A, Tsokos GC. Cyclic adenosine 5′-monophosphate response element modulator is responsible for the decreased expression of c-fos and activator protein-1 binding in T cells from patients with systemic lupus erythematosus. J Immunol. 2004; 173:3557–3563.

153. Crispín JC, Oukka M, Bayliss G, Cohen RA, Van Beek CA, Stillman IE, et al. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol. 2008; 181:8761–8766.

154. Crispín JC, Tsokos GC. Human TCR-alpha beta+ CD4−CD8− T cells can derive from CD8+ T cells and display an inflammatory effector phenotype. J Immunol. 2009; 183:4675–4681.
155. Shivakumar S, Tsokos GC, Datta SK. T cell receptor alpha/beta expressing double-negative (CD4−/CD8−) and CD4+ T helper cells in humans augment the production of pathogenic anti-DNA autoantibodies associated with lupus nephritis. J Immunol. 1989; 143:103–112.
156. Apostolidis SA, Crispín JC, Tsokos GC. IL-17-producing T cells in lupus nephritis. Lupus. 2011; 20:120–124.

157. Winchester R, Wiesendanger M, Zhang HZ, Steshenko V, Peterson K, Geraldino-Pardilla L, et al. Immunologic characteristics of intrarenal T cells: trafficking of expanded CD8+ T cell β-chain clonotypes in progressive lupus nephritis. Arthritis Rheum. 2012; 64:1589–1600.

158. Enghard P, Rieder C, Kopetschke K, Klocke JR, Undeutsch R, Biesen R, et al. Urinary CD4 T cells identify SLE patients with proliferative lupus nephritis and can be used to monitor treatment response. Ann Rheum Dis. 2014; 73:277–283.

159. Mandik-Nayak L, Seo SJ, Sokol C, Potts KM, Bui A, Erikson J. MRL-lpr/lpr mice exhibit a defect in maintaining developmental arrest and follicular exclusion of anti-double-stranded DNA B cells. J Exp Med. 1999; 189:1799–1814.

160. Sinai P, Dozmorov IM, Song R, Schwartzberg PL, Wakeland EK, Wülfing C. T/B-cell interactions are more transient in response to weak stimuli in SLE-prone mice. Eur J Immunol. 2014; 44:3522–3531.

161. Choi JY, Ho JH, Pasoto SG, Bunin V, Kim ST, Carrasco S, et al. Circulating follicular helper-like T cells in systemic lupus erythematosus: association with disease activity. Arthritis Rheumatol. 2015; 67:988–999.

162. Szabó K, Papp G, Szántó A, Tarr T, Zeher M. A comprehensive investigation on the distribution of circulating follicular T helper cells and B cell subsets in primary Sjögren's syndrome and systemic lupus erythematosus. Clin Exp Immunol. 2016; 183:76–89.

163. Le Coz C, Joublin A, Pasquali JL, Korganow AS, Dumortier H, Monneaux F. Circulating TFH subset distribution is strongly affected in lupus patients with an active disease. PLoS One. 2013; 8:e75319.

164. Jacquemin C, Schmitt N, Contin-Bordes C, Liu Y, Narayanan P, Seneschal J, et al. OX40 Ligand Contributes to Human Lupus Pathogenesis by Promoting T Follicular Helper Response. Immunity. 2015; 42:1159–1170.

165. Cortini A, Ellinghaus U, Malik TH, Cunninghame Graham DS, Botto M, Vyse TJ. B cell OX40L supports T follicular helper cell development and contributes to SLE pathogenesis. Ann Rheum Dis. 2017; 76:2095–2103.

166. Simpson N, Gatenby PA, Wilson A, Malik S, Fulcher DA, Tangye SG, et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 2010; 62:234–244.

167. Floege J, Eitner F, Alpers CE. A new look at platelet-derived growth factor in renal disease. J Am Soc Nephrol. 2008; 19:12–23.

168. Fu J, Lee K, Chuang PY, Liu Z, He JC. Glomerular endothelial cell injury and cross talk in diabetic kidney disease. Am J Physiol Renal Physiol. 2015; 308:F287–F297.

169. Daehn I, Casalena G, Zhang T, Shi S, Fenninger F, Barasch N, et al. Endothelial mitochondrial oxidative stress determines podocyte depletion in segmental glomerulosclerosis. J Clin Invest. 2014; 124:1608–1621.

170. Schrimpf C, Teebken OE, Wilhelmi M, Duffield JS. The role of pericyte detachment in vascular rarefaction. J Vasc Res. 2014; 51:247–258.

171. Dimke H, Sparks MA, Thomson BR, Frische S, Coffman TM, Quaggin SE. Tubulovascular cross-talk by vascular endothelial growth factor a maintains peritubular microvasculature in kidney. J Am Soc Nephrol. 2015; 26:1027–1038.

172. Kida Y, Ieronimakis N, Schrimpf C, Reyes M, Duffield JS. EphrinB2 reverse signaling protects against capillary rarefaction and fibrosis after kidney injury. J Am Soc Nephrol. 2013; 24:559–572.

173. Kümpers P, David S, Haubitz M, Hellpap J, Horn R, Bröcker V, et al. The Tie2 receptor antagonist angiopoietin 2 facilitates vascular inflammation in systemic lupus erythematosus. Ann Rheum Dis. 2009; 68:1638–1643.
174. Kida Y, Tchao BN, Yamaguchi I. Peritubular capillary rarefaction: a new therapeutic target in chronic kidney disease. Pediatr Nephrol. 2014; 29:333–342.

175. Gilkeson GS, Mashmoushi AK, Ruiz P, Caza TN, Perl A, Oates JC. Endothelial nitric oxide synthase reduces crescentic and necrotic glomerular lesions, reactive oxygen production, and MCP1 production in murine lupus nephritis. PLoS One. 2013; 8:e64650.

176. Van Linthout S, Miteva K, Tschöpe C. Crosstalk between fibroblasts and inflammatory cells. Cardiovasc Res. 2014; 102:258–269.

177. Duffield JS. Cellular and molecular mechanisms in kidney fibrosis. J Clin Invest. 2014; 124:2299–2306.

178. Deapen D, Escalante A, Weinrib L, Horwitz D, Bachman B, Roy-Burman P, et al. A revised estimate of twin concordance in systemic lupus erythematosus. Arthritis Rheum. 1992; 35:311–318.
179. Nishino H, Shibuya K, Nishida Y, Mushimoto M. Lupus erythematosus-like syndrome with selective complete deficiency of C1q. Ann Intern Med. 1981; 95:322–324.

180. Hannema AJ, Kluin-Nelemans JC, Hack CE, Eerenberg-Belmer AJ, Mallée C, van Helden HP. SLE like syndrome and functional deficiency of C1q in members of a large family. Clin Exp Immunol. 1984; 55:106–114.
181. Botto M, Dell'Agnola C, Bygrave AE, Thompson EM, Cook HT, Petry F, et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998; 19:56–59.

182. Botto M, Kirschfink M, Macor P, Pickering MC, Würzner R, Tedesco F. Complement in human diseases: Lessons from complement deficiencies. Mol Immunol. 2009; 46:2774–2783.

183. Niu Z, Zhang P, Tong Y. Value of HLA-DR genotype in systemic lupus erythematosus and lupus nephritis: a meta-analysis. Int J Rheum Dis. 2015; 18:17–28.

184. Kim K, Bang SY, Yoo DH, Cho SK, Choi CB, Sung YK, et al. Imputing Variants in HLA-DR beta genes reveals that HLA-DRB1 is solely associated with rheumatoid arthritis and systemic lupus erythematosus. PLoS One. 2016; 11:e0150283.

185. Kim-Howard X, Maiti AK, Anaya JM, Bruner GR, Brown E, Merrill JT, et al. ITGAM coding variant (rs1143679) influences the risk of renal disease, discoid rash and immunological manifestations in patients with systemic lupus erythematosus with European ancestry. Ann Rheum Dis. 2010; 69:1329–1332.

186. Dong C, Ptacek TS, Redden DT, Zhang K, Brown EE, Edberg JC, et al. Fcγ receptor IIIa single-nucleotide polymorphisms and haplotypes affect human IgG binding and are associated with lupus nephritis in African Americans. Arthritis Rheumatol. 2014; 66:1291–1299.

187. Faridi MH, Khan SQ, Zhao W, Lee HW, Altintas MM, Zhang K, et al. CD11b activation suppresses TLR-dependent inflammation and autoimmunity in systemic lupus erythematosus. J Clin Invest. 2017; 127:1271–1283.

188. International MHC, Rioux JD, Goyette P, Vyse TJ, Hammarström L, Fernando MM, et al. Mapping of multiple susceptibility variants within the MHC region for 7 immune-mediated diseases. Proc Natl Acad Sci U S A. 2009; 106:18680–18685.
189. Bentham J, Morris DL, Graham DSC, Pinder CL, Tombleson P, Behrens TW, et al. Genetic association analyses implicate aberrant regulation of innate and adaptive immunity genes in the pathogenesis of systemic lupus erythematosus. Nat Genet. 2015; 47:1457–1464.

190. Niewold TB, Kelly JA, Flesch MH, Espinoza LR, Harley JB, Crow MK. Association of the IRF5 risk haplotype with high serum interferon-alpha activity in systemic lupus erythematosus patients. Arthritis Rheum. 2008; 58:2481–2487.
191. Kariuki SN, Franek BS, Kumar AA, Arrington J, Mikolaitis RA, Utset TO, et al. Trait-stratified genome-wide association study identifies novel and diverse genetic associations with serologic and cytokine phenotypes in systemic lupus erythematosus. Arthritis Res Ther. 2010; 12:R151.

192. Ramos PS, Williams AH, Ziegler JT, Comeau ME, Guy RT, Lessard CJ, et al. Genetic analyses of interferon pathway-related genes reveal multiple new loci associated with systemic lupus erythematosus. Arthritis Rheum. 2011; 63:2049–2057.

193. Boone DL, Turer EE, Lee EG, Ahmad RC, Wheeler MT, Tsui C, et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol. 2004; 5:1052–1060.

194. de Jong TD, Vosslamber S, Mantel E, de Ridder S, Wesseling JG, van der Pouw Kraan TC, et al. Physiological evidence for diversification of IFNα- and IFNβ-mediated response programs in different autoimmune diseases. Arthritis Res Ther. 2016; 18:49.

195. Deng Y, Tsao BP. Advances in lupus genetics and epigenetics. Curr Opin Rheumatol. 2014; 26:482–492.

196. Lessard CJ, Sajuthi S, Zhao J, Kim K, Ice JA, Li H, et al. Identification of a systemic lupus erythematosus risk locus spanning ATG16L2, FCHSD2, and P2RY2 in Koreans. Arthritis Rheumatol. 2016; 68:1197–1209.
197. Sun C, Molineros JE, Looger LL, Zhou XJ, Kim K, Okada Y, et al. High-density genotyping of immune-related loci identifies new SLE risk variants in individuals with Asian ancestry. Nat Genet. 2016; 48:323–330.

198. Olsson LM, Johansson ÅC, Gullstrand B, Jönsen A, Saevarsdottir S, Rönnblom L, et al. A single nucleotide polymorphism in the NCF1 gene leading to reduced oxidative burst is associated with systemic lupus erythematosus. Ann Rheum Dis. 2017; 76:1607–1613.
199. An J, Briggs TA, Dumax-Vorzet A, Alarcón-Riquelme ME, Belot A, Beresford M, et al. Tartrate-Resistant Acid Phosphatase Deficiency in the Predisposition to Systemic Lupus Erythematosus. Arthritis Rheumatol. 2017; 69:131–142.

200. Kim SJ, Schätzle S, Ahmed SS, Haap W, Jang SH, Gregersen PK, et al. Increased cathepsin S in Prdm1-/- dendritic cells alters the TFH cell repertoire and contributes to lupus. Nat Immunol. 2017; 18:1016–1024.

201. International Consortium for Systemic Lupus Erythematosus Genetics (SLEGEN). Harley JB, Alarcón-Riquelme ME, Criswell LA, Jacob CO, Kimberly RP, et al. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat Genet. 2008; 40:204–210.

202. Raffler J, Friedrich N, Arnold M, Kacprowski T, Rueedi R, Altmaier E, et al. Genome-wide association study with targeted and non-targeted NMR metabolomics identifies 15 novel loci of urinary human metabolic individuality. PLoS Genet. 2015; 11:e1005487.

203. Tsai LJ, Hsiao SH, Tsai LM, Lin CY, Tsai JJ, Liou DM, et al. The sodium-dependent glucose cotransporter SLC5A11 as an autoimmune modifier gene in SLE. Tissue Antigens. 2008; 71:114–126.

204. Caster DJ, Korte EA, Nanda SK, McLeish KR, Oliver RK, G'sell RT, et al. ABIN1 dysfunction as a genetic basis for lupus nephritis. J Am Soc Nephrol. 2013; 24:1743–1754.
205. Sanchez E, Nadig A, Richardson BC, Freedman BI, Kaufman KM, Kelly JA, et al. Phenotypic associations of genetic susceptibility loci in systemic lupus erythematosus. Ann Rheum Dis. 2011; 70:1752–1757.

206. Bolin K, Sandling JK, Zickert A, Jönsen A, Sjöwall C, Svenungsson E, et al. Association of STAT4 polymorphism with severe renal insufficiency in lupus nephritis. PLoS One. 2013; 8:e84450.

207. Liu K, Li QZ, Delgado-Vega AM, Abelson AK, Sánchez E, Kelly JA, et al. Kallikrein genes are associated with lupus and glomerular basement membrane-specific antibody-induced nephritis in mice and humans. J Clin Invest. 2009; 119:911–923.
208. Lee YH, Bae SC. Association between the functional ITGAM rs1143679 G/A polymorphism and systemic lupus erythematosus/lupus nephritis or rheumatoid arthritis: an update meta-analysis. Rheumatol Int. 2015; 35:815–823.

209. Sawalha AH, Jeffries M, Webb R, Lu Q, Gorelik G, Ray D, et al. Defective T-cell ERK signaling induces interferon-regulated gene expression and overexpression of methylation-sensitive genes similar to lupus patients. Genes Immun. 2008; 9:368–378.

210. Coit P, Renauer P, Jeffries MA, Merrill JT, McCune WJ, Maksimowicz-McKinnon K, et al. Renal involvement in lupus is characterized by unique DNA methylation changes in naïve CD4+ T cells. J Autoimmun. 2015; 61:29–35.

211. Coit P, Dozmorov MG, Merrill JT, McCune WJ, Maksimowicz-McKinnon K, Wren JD, et al. Epigenetic Reprogramming in Naive CD4+ T Cells Favoring T Cell Activation and Non-Th1 Effector T Cell Immune Response as an Early Event in Lupus Flares. Arthritis Rheumatol. 2016; 68:2200–2209.

212. Yang Y, Tang Q, Zhao M, Liang G, Wu H, Li D, et al. The effect of mycophenolic acid on epigenetic modifications in lupus CD4+T cells. Clin Immunol. 2015; 158:67–76.

213. Zhang Z, Song L, Maurer K, Petri MA, Sullivan KE. Global H4 acetylation analysis by ChIP-chip in systemic lupus erythematosus monocytes. Genes Immun. 2010; 11:124–133.

214. Dai Y, Huang YS, Tang M, Lv TY, Hu CX, Tan YH, et al. Microarray analysis of microRNA expression in peripheral blood cells of systemic lupus erythematosus patients. Lupus. 2007; 16:939–946.

215. Dai Y, Sui W, Lan H, Yan Q, Huang H, Huang Y. Comprehensive analysis of microRNA expression patterns in renal biopsies of lupus nephritis patients. Rheumatol Int. 2009; 29:749–754.

216. Costa-Reis P, Russo PA, Zhang Z, Colonna L, Maurer K, Gallucci S, et al. The Role of MicroRNAs and human epidermal growth factor receptor 2 in proliferative lupus nephritis. Arthritis Rheumatol. 2015; 67:2415–2426.

217. Carlsen AL, Schetter AJ, Nielsen CT, Lood C, Knudsen S, Voss A, et al. Circulating microRNA expression profiles associated with systemic lupus erythematosus. Arthritis Rheum. 2013; 65:1324–1334.

218. Yan S, Yim LY, Lu L, Lau CS, Chan VS. MicroRNA regulation in systemic lupus erythematosus pathogenesis. Immune Netw. 2014; 14:138–148.

219. Nelson P, Rylance P, Roden D, Trela M, Tugnet N. Viruses as potential pathogenic agents in systemic lupus erythematosus. Lupus. 2014; 23:596–605.

220. Sawalha AH, Schmid WR, Binder SR, Bacino DK, Harley JB. Association between systemic lupus erythematosus and Helicobacter pylori seronegativity. J Rheumatol. 2004; 31:1546–1550.
221. Ram M, Anaya JM, Barzilai O, Izhaky D, Porat Katz BS, Blank M, et al. The putative protective role of hepatitis B virus (HBV) infection from autoimmune disorders. Autoimmun Rev. 2008; 7:621–625.

222. Chen M, Aosai F, Norose K, Mun HS, Ishikura H, Hirose S, et al. Toxoplasma gondii infection inhibits the development of lupus-like syndrome in autoimmune (New Zealand Black x New Zealand White) F1 mice. Int Immunol. 2004; 16:937–946.

223. Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol. 2005; 23:307–336.
224. Zandman-Goddard G, Berkun Y, Barzilai O, Boaz M, Blank M, Ram M, et al. Exposure to Epstein-Barr virus infection is associated with mild systemic lupus erythematosus disease. Ann N Y Acad Sci. 2009; 1173:658–663.

225. Shi L, Zhang Z, Yu AM, Wang W, Wei Z, Akhter E, et al. The SLE transcriptome exhibits evidence of chronic endotoxin exposure and has widespread dysregulation of noncoding and coding RNAs. PLoS One. 2014; 9:e93846.

226. Nockher WA, Wigand R, Schoeppe W, Scherberich JE. Elevated levels of soluble CD14 in serum of patients with systemic lupus erythematosus. Clin Exp Immunol. 1994; 96:15–19.
227. Gallo PM, Rapsinski GJ, Wilson RP, Oppong GO, Sriram U, Goulian M, et al. Amyloid-DNA Composites of Bacterial Biofilms Stimulate Autoimmunity. Immunity. 2015; 42:1171–1184.

228. Wagner H. Bacterial CpG DNA activates immune cells to signal infectious danger. Adv Immunol. 1999; 73:329–368.

229. Lövgren T, Eloranta ML, Båve U, Alm GV, Rönnblom L. Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum. 2004; 50:1861–1872.
230. Means TK, Latz E, Hayashi F, Murali MR, Golenbock DT, Luster AD. Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. J Clin Invest. 2005; 115:407–417.

231. Hevia A, Milani C, López P, Cuervo A, Arboleya S, Duranti S, et al. Intestinal dysbiosis associated with systemic lupus erythematosus. MBio. 2014; 5:e01548–e01514.

232. Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013; 504:446–450.

233. Arpaia N, Campbell C, Fan X, Dikiy S, van der, deRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013; 504:451–455.

234. Pawar RD, Castrezana-Lopez L, Allam R, Kulkarni OP, Segerer S, Radomska E, et al. Bacterial lipopeptide triggers massive albuminuria in murine lupus nephritis by activating Toll-like receptor 2 at the glomerular filtration barrier. Immunology. 2009; 128:1 Suppl. e206–e221.

235. Achtman JC, Werth VP. Pathophysiology of cutaneous lupus erythematosus. Arthritis Res Ther. 2015; 17:182.

236. Caricchio R, McPhie L, Cohen PL. Ultraviolet B radiation-induced cell death: critical role of ultraviolet dose in inflammation and lupus autoantigen redistribution. J Immunol. 2003; 171:5778–5786.

237. Wu Z, Li X, Qin H, Zhu X, Xu J, Shi W. Ultraviolet B enhances DNA hypomethylation of CD4+ T cells in systemic lupus erythematosus via inhibiting DNMT1 catalytic activity. J Dermatol Sci. 2013; 71:167–173.

238. Zhu X, Liang J, Li F, Yang Y, Xiang L, Xu J. Analysis of associations between the patterns of global DNA hypomethylation and expression of DNA methyltransferase in patients with systemic lupus erythematosus. Int J Dermatol. 2011; 50:697–704.

239. Reefman E, Kuiper H, Limburg PC, Kallenberg CG, Bijl M. Type I interferons are involved in the development of ultraviolet B-induced inflammatory skin lesions in systemic lupus erythaematosus patients. Ann Rheum Dis. 2008; 67:11–18.

240. Cornacchia E, Golbus J, Maybaum J, Strahler J, Hanash S, Richardson B. Hydralazine and procainamide inhibit T cell DNA methylation and induce autoreactivity. J Immunol. 1988; 140:2197–2200.
241. Richardson B, Scheinbart L, Strahler J, Gross L, Hanash S, Johnson M. Evidence for impaired T cell DNA methylation in systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum. 1990; 33:1665–1673.

242. Dorgham K, Amoura Z, Parizot C, Arnaud L, Frances C, Pionneau C, et al. Ultraviolet light converts propranolol, a nonselective β-blocker and potential lupus-inducing drug, into a proinflammatory AhR ligand. Eur J Immunol. 2015; 45:3174–3187.

243. Finckh A, Cooper GS, Chibnik LB, Costenbader KH, Watts J, Pankey H, et al. Occupational silica and solvent exposures and risk of systemic lupus erythematosus in urban women. Arthritis Rheum. 2006; 54:3648–3654.

244. Barbhaiya M, Tedeschi SK, Lu B, Malspeis S, Kreps D, Sparks JA, et al. Cigarette smoking and the risk of systemic lupus erythematosus, overall and by anti-double stranded DNA antibody subtype, in the Nurses' Health Study cohorts. Ann Rheum Dis. 2018; 77:196–202.

245. Young KA, Munroe ME, Guthridge JM, Kamen DL, Niewold TB, Gilkeson GS, et al. Combined role of vitamin D status and CYP24A1 in the transition to systemic lupus erythematosus. Ann Rheum Dis. 2017; 76:153–158.

246. Barbhaiya M, Lu B, Sparks JA, Malspeis S, Chang SC, Karlson EW, et al. Influence of alcohol consumption on the risk of systemic lupus erythematosus among women in the nurses' health study cohorts. Arthritis Care Res (Hoboken). 2017; 69:384–392.

247. Dayan M, Zinger H, Kalush F, Mor G, Amir-Zaltzman Y, Kohen F, et al. The beneficial effects of treatment with tamoxifen and anti-oestradiol antibody on experimental systemic lupus erythematosus are associated with cytokine modulations. Immunology. 1997; 90:101–108.

248. Roubinian JR, Talal N, Greenspan JS, Goodman JR, Siiteri PK. Effect of castration and sex hormone treatment on survival, anti-nucleic acid antibodies, and glomerulonephritis in NZB/NZW F1 mice. J Exp Med. 1978; 147:1568–1583.

249. Roubinian J, Talal N, Siiteri PK, Sadakian JA. Sex hormone modulation of autoimmunity in NZB/NZW mice. Arthritis Rheum. 1979; 22:1162–1169.

250. Bynoe MS, Grimaldi CM, Diamond B. Estrogen up-regulates Bcl-2 and blocks tolerance induction of naive B cells. Proc Natl Acad Sci U S A. 2000; 97:2703–2708.

251. Grimaldi CM, Michael DJ, Diamond B. Cutting edge: expansion and activation of a population of autoreactive marginal zone B cells in a model of estrogen-induced lupus. J Immunol. 2001; 167:1886–1890.

252. Grimaldi CM. Sex and systemic lupus erythematosus: the role of the sex hormones estrogen and prolactin on the regulation of autoreactive B cells. Curr Opin Rheumatol. 2006; 18:456–461.

253. Grimaldi CM, Cleary J, Dagtas AS, Moussai D, Diamond B. Estrogen alters thresholds for B cell apoptosis and activation. J Clin Invest. 2002; 109:1625–1633.

254. Hartley SB, Cooke MP, Fulcher DA, Harris AW, Cory S, Basten A, et al. Elimination of self-reactive B lymphocytes proceeds in two stages: arrested development and cell death. Cell. 1993; 72:325–335.

255. Treadwell EL, Wiley K, Word B, Melchior W, Tolleson WH, Gopee N, et al. Prolactin and Dehydroepiandrosterone Levels in Women with Systemic Lupus Erythematosus: The Role of the Extrapituitary Prolactin Promoter Polymorphism at -1149G/T. J Immunol Res. 2015; 2015:435658.

256. Lahita RG, Kunkel HG, Bradlow HL. Increased oxidation of testosterone in systemic lupus erythematosus. Arthritis Rheum. 1983; 26:1517–1521.

257. Lahita RG, Bradlow HL, Ginzler E, Pang S, New M. Low plasma androgens in women with systemic lupus erythematosus. Arthritis Rheum. 1987; 30:241–248.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download