Abstract
Objective
Methods
Results
REFERENCES
Figure 1.
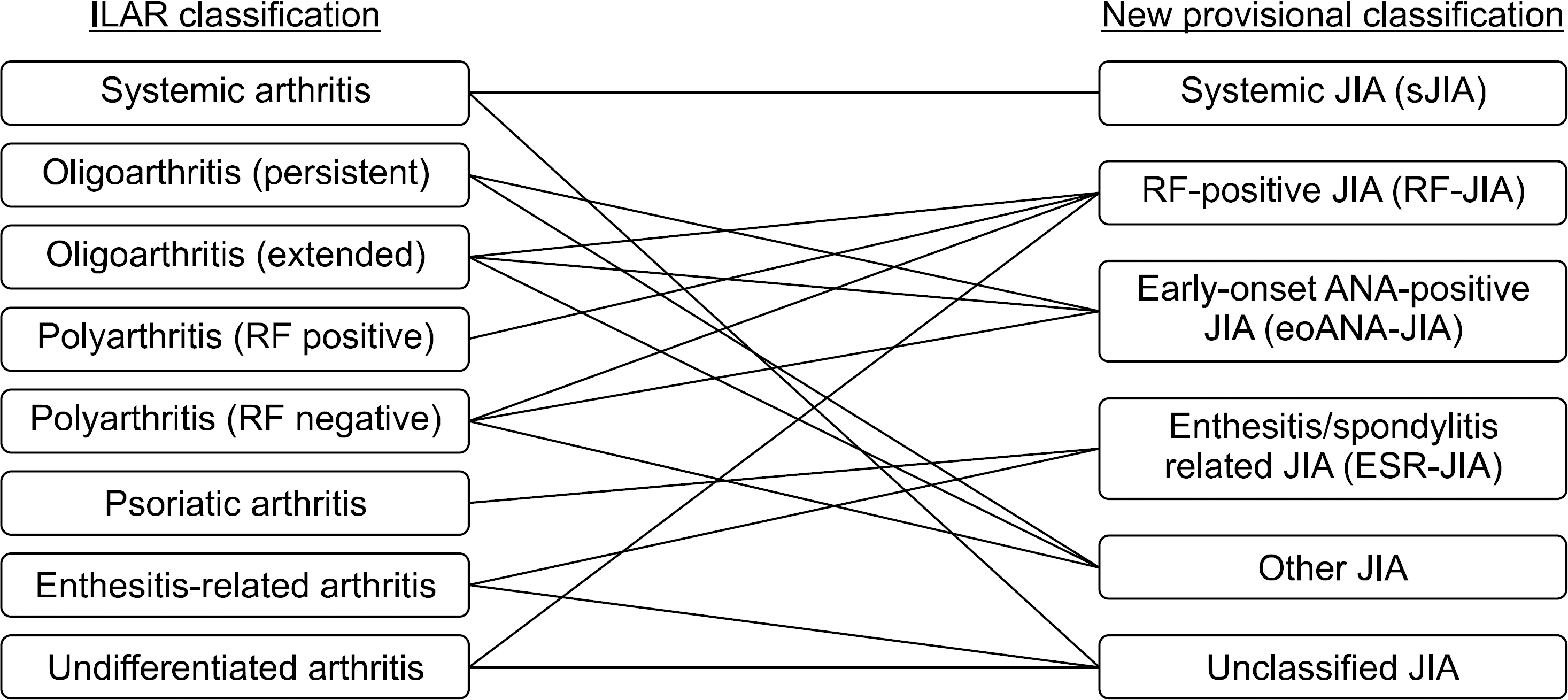
Table 1.
Except where indicated otherwise, values are the number (%). JIA: juvenile idiopathic arthritis, ILAR: International League of Associations for Rheumatology, PE: persistent, EX: extended, RF: rheumatoid factor, ERA: enthesitis-related arthritis, IQR: interquartile range, Anti-CCP Ab: anti-cyclic citrullinated peptide antibody, ANA: antinuclear antibody, HLA: human leukocyte antigen, SD: standard deviation.
Table 2.
| Variable | sJIA | RF-JIA | eoANA-JIA | ESR-JIA | Other JIA | Unclassified JIA | Total | p-value∗ | Comparisons significant on post-hoc tests† |
|---|---|---|---|---|---|---|---|---|---|
| Patient ILAR subtypes (n) | 71 (27.1) | 31 (11.8) | 22 (8.4) | 63 (24.0) | 65 (24.8) | 10 (3.8) | 262 (100) | ||
| Systemic arthritis | 71 | 0 | 0 | 0 | 0 | 2 | 73 | ||
| Oligoarthritis PE | 0 | 0 | 12 | 0 | 27 | 0 | 39 | ||
| Oligoarthritis EX | 0 | 2 | 3 | 0 | 11 | 0 | 16 | ||
| Polyarthritis RF (−) | 0 | 3 | 7 | 0 | 27 | 0 | 37 | ||
| Polyarthritis RF (+) Psoriatic arthritis | 0 0 | 15 0 | 0 0 | 0 1 | 0 0 | 0 0 | 15 1 | ||
| ERA | 0 | 0 | 0 | 62 | 0 | 1 | 63 | ||
| Undifferentiated | 0 | 11 | 0 | 0 | 0 | 7 | 18 | ||
| Male: Female (female, %) | 38:33 (46.5) | 7:24 (77.4) | 5:17 (77.3) | 56:7 (11.1) | 20:45 (69.2) | 6:4 (40.0) | 132:130 (49.6) | <0.00001 | RF vs. ESR ANA vs. ESR |
| Disease onset, median (IQR) (yr) | 6.0 (3.8∼9.3) | 7.0 (4.0∼9.6) | 2.4 (2.1∼4.2) | ) 9.8 (7.8∼11.8) | 5.6 (3.0∼10.9) | 9.0 (5.2∼12.2) | 7.3 (3.9∼10.5) | <0.00001 | ANA vs. RF RF vs. ESR |
| ANA vs. ESR | |||||||||
| RF | 0 (0) | 26 (83.9) | 0 (0) | 0 (0) | 0 (0) | 3 (30.0) | 29 (11.1) | ||
| Anti-CCP Ab | 0 (0) | 27 (87.1) | 0 (0) | 0 (0) | 0 (0) | 4 (40.0) | 31 (12.7) | ||
| ANA | 12 (16.9) | 19 (61.3) | 22 (100) | 8 (12.7) | 11 (16.9) | 2 (20.0) | 74 (28.2) | ||
| HLA-B27 | 5 (7.4) | 5 (17.2) | 4 (20.0) | 57 (90.5) | 7 (10.8) | 6 (60.0) | 84 (32.9) | <0.00001 | RF vs. ESR |
| ANA vs. ESR | |||||||||
| Uveitis | 7 (9.9) | 2 (6.5) | 7 (31.8) | 9 (14.3) | 7 (10.8) | 0 (0) | 32 (12.2) | 0.039 | |
| Enthesitis | 1 (1.4) | 0 (0) | 0 (0) | 31 (49.2) | 3 (4.6) | 2 (20.0) | 37 (14.1) | <0.00001 | RF vs. ESR |
| Symmetricity | 34 (47.9) | 19 (61.3) | 5 (22.7) | 14 (22.2) | 38 (58.5) | 7 (70.0) | 117 (44.7) | 0.0004 | ANA vs. ERA RF vs. ESR |
| Cumulative no. of joints involved, mean±SD | 7.4±9.3 | 14.2±7.9 | 6.0±6.3 | 6.1±6.2 | 8.0±7.2 | 8.8±9.4 | 8.0±8.0 | <0.00001 | ANA vs. RF ANA vs. RF RF vs. ERA |
| Upper large joint | 35 (49.3) | 29 (93.5) | 12 (54.5) | 17 (27.0) | 35 (53.8) | 6 (60.0) | 134 (51.1) | <0.00001 | RF vs. ESR |
| Upper small joint | 20 (28.2) | 25 (80.6) | 9 (40.9) | 18 (28.6) | 26 (40.0) | 4 (40.0) | 102 (38.9) | 0.00001 | ANA vs. RF RF vs. ESR |
| Lower large joint | 59 (83.1) | 27 (87.1) | 19 (86.4) | 58 (92.1) | 59 (90.8) | 8 (80.0) | 230 (87.8) | 0.647 | ANA vs. RF |
| Lower small joint | 12 (16.9) | 16 (51.6) | 9 (27.3) | 26 (41.3) | 15 (23.1) | 3 (30.0) | 78 (29.8) | 0.208 | |
| Axial joint | 20 (28.2) | 16 (51.6) | 3 (13.6) | 35 (55.6) | 27 (41.5) | 4 (40.0) | 105 (40.1) | 0.003 | ANA vs. ESR |
| ANA vs. RF | |||||||||
| ASAS criteria | 9 (12.7) | 6 (19.4) | 10 (45.5) | 62 (98.4) | 14 (21.5) | 6 (60.0) | 107 (40.8) | <0.00001 | RF vs. ESR |
| ANA vs. ESR | |||||||||
| Axial SpA (n) | 0 | 0 | 0 | 9 | 1 | 1 | 11 | ||
| Peripheral SpA (n) | 9 | 6 | 10 | 53 | 13 | 5 | 96 |
Except where indicated otherwise, values are the number (%). JIA: juvenile idiopathic arthritis, sJIA: systemic JIA, RF-JIA: Rheumatoid factor-positive JIA, eoANA-JIA: early-onset antinuclear antibody-positive JIA, ESR-JIA: enthesitis/spondylitis-related JIA, ILAR: International League of Associations for Rheumatology, PE: persistent, EX: extended, ERA: enthesitis-related arthritis, IQR: interquartile range, Anti-CCP Ab: anti-cyclic citrullinated peptide antibody, ANA: antinuclear antibody, HLA: human leukocyte antigen, SD: standard deviation, ASAS: Assessment of SpondyloArthritis International Society, SpA: spondyloarthritis.
* For overall comparisons. Following three subtypes were compared statistically—RF positive arthritis, ANA positive arthritis, and ERA/SpA. Comparisons of quantitative data were made by Mann-Whitney U test; comparisons of frequencies were made by chi-square test (or by Fishers exact test if expected frequencies were <5).
Table 3.
| Variable | RF-JIA | eoANA-JIA | ESR-JIA | p-value∗ | Comparisons significant on post-hoc test† |
|---|---|---|---|---|---|
| Lower limbs Knee | 23 (74.2) | 18 (81.8) | 51 (81.0) | 0.711 | |
| Ankle | 25 (80.6) | 11 (50.0) | 37 (58.7) | 0.045 | |
| MTP joint | 15 (48.4) | 4 (18.2) | 18 (28.6) | 0.047 | |
| Toe | 8 (25.8) | 3 (13.6) | 16 (25.4) | 0.493 | |
| Upper limbs | |||||
| Elbow | 14 (45.2) | 8 (36.4) | 7 (11.1) | 0.001 | RF vs. ESR |
| Wrist | 28 (93.3) | 7 (31.8) | 13 (20.6) | <0.00001 | ANA vs. RF |
| MCP joint | 14 (45.2) | 5 (22.7) | 13 (20.6) | 0.037 | RF vs. ESR RF vs. ESR |
| Finger | 21 (67.7) | 8 (36.4) | 8 (12.7) | <0.00001 | RF vs. ESR |
| Axial joints | |||||
| TMJ | 4 (12.9) | 2 (9.1) | 4 (6.3) | 0.565 | |
| Neck | 1 (3.2) | 1 (3.0) | 8 (12.7) | 0.087 | |
| Shoulder | 11 (35.5) | 0 (0) | 11 (17.5) | 0.005 | ANA vs. RF |
| Back | 0 (0) | 0 (0) | 10 (15.9) | 0.010 | |
| Sacroiliac joint | 1 (3.2) | 0 (0) | 9 (14.3) | 0.055 | |
| Hip | 12 (38.7) | 2 (9.1) | 26 (41.3) | 0.020 | ANA vs. RF |
| ANA vs. ESR |
Except where indicated otherwise, values are the number (%). Comparisons of frequencies were made by chi-square test (or by Fisher's exact test if expected frequencies were <5). RF: rheumatoid factor, ANA: antinuclear antibody, MTP: metatarsophalangeal, MCP: metacarpophalangeal, TMJ: temporomandibular joint, ERA: enthesitis-related arthritis, SpA: spondyloarthritis.
* For overall comparisons. Following three subtypes were compared statistically― RF positive arthritis, ANA positive arthritis, and ERA/SpA. Comparisons of quantitative data were made by Mann-Whitney U test; comparisons of frequencies were made by chi-square test (or by Fisher's exact test if expected frequencies were <5).
Table 4.
Table 5.
Except where indicated otherwise, values are the number (%). Comparisons of quantitative data were made by Mann-Whitney U test; comparisons of frequencies were made by chi-square test (or by Fisher's exact test if expected frequencies were <5). ANA: antinuclear antibody, IQR: interquartile range, HLA: human leukocyte antigen, SD: standard deviation.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download