Abstract
The mean serum uric acid level and the prevalence of hyperuricemia have increased over the past few decades. Hyperuricemia is considered to be responsible for the increased risk of hypertension, insulin resistance, and cardiovascular disease. Metabolic syndrome also contributes to the development of type II diabetes mellitus and cardiovascular disease. Despite the close relationships between uric acid and the components of metabolic syndrome, the causal effect of uric acid on these clinical issues is unclear. Recent studies have revealed the possible development of metabolic syndrome mediated by fructose-induced hyperuricemia. This review summarizes the available clinical and experimental data supporting the causal effect of uric acid on the components of metabolic syndrome, including hypertension, cardiovascular disease, and insulin resistance.
Go to : 
REFERENCES
1. Choi HK, Mount DB, Reginato AM. American College of Physicians; American Physiological Society. Pathogenesis of gout. Ann Intern Med. 2005; 143:499–516.

2. Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981; 78:6858–62.

3. Sautin YY, Nakagawa T, Zharikov S, Johnson RJ. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol. 2007; 293:C584–96.

4. Kim KY, Ralph Schumacher H, Hunsche E, Wertheimer AI, Kong SX. A literature review of the epidemiology and treatment of acute gout. Clin Ther. 2003; 25:1593–617.

5. Johnson RJ, Kang DH, Feig D, Kivlighn S, Kanellis J, Watanabe S, et al. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension. 2003; 41:1183–90.

6. Sarafidis PA, Nilsson PM. The metabolic syndrome: a glance at its history. J Hypertens. 2006; 24:621–6.

7. Liberopoulos EN, Mikhailidis DP, Elisaf MS. Diagnosis and management of the metabolic syndrome in obesity. Obes Rev. 2005; 6:283–96.

8. Daskalopoulou SS, Mikhailidis DP, Elisaf M. Prevention and treatment of the metabolic syndrome. Angiology. 2004; 55:589–612.

9. Choi HK, Ford ES. Prevalence of the metabolic syndrome in individuals with hyperuricemia. Am J Med. 2007; 120:442–7.

10. Sui X, Church TS, Meriwether RA, Lobelo F, Blair SN. Uric acid and the development of metabolic syndrome in women and men. Metabolism. 2008; 57:845–52.

11. Ryu S, Song J, Choi BY, Lee SJ, Kim WS, Chang Y, et al. Incidence and risk factors for metabolic syndrome in Korean male workers, ages 30 to 39. Ann Epidemiol. 2007; 17:245–52.

12. Jung JH, Song GG, Ji JD, Lee YH, Kim JH, Seo YH, et al. Metabolic syndrome: prevalence and risk factors in Korean gout patients. Korean J Intern Med. 2016 Oct 12; [Epub].DOI: DOI: 10.3904/kjim.2016.062.

13. Yoo HG, Lee SI, Chae HJ, Park SJ, Lee YC, Yoo WH. Prevalence of insulin resistance and metabolic syndrome in patients with gouty arthritis. Rheumatol Int. 2011; 31:485–91.

14. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998; 15:539–53.

15. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the national cholesterol education program (NCEP) Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001; 285:2486–97.
16. Alberti KG, Zimmet P, Shaw J. IDF Epidemiology Task Force Consensus Group. The metabolic syndrome–a new worldwide definition. Lancet. 2005; 366:1059–62.

17. Chen LY, Zhu WH, Chen ZW, Dai HL, Ren JJ, Chen JH, et al. Relationship between hyperuricemia and metabolic syndrome. J Zhejiang Univ Sci B. 2007; 8:593–8.

18. López-Suárez A, Elvira-González J, Bascuñana-Quirell A, Rosal-Obrador J, Michán-Doña A, Escribano-Serrano J, et al. Serum urate levels and urinary uric acid excretion in subjects with metabolic syndrome. Med Clin (Barc). 2006; 126:321–4.
19. Hjortnaes J, Algra A, Olijhoek J, Huisman M, Jacobs J, van der Graaf Y, et al. Serum uric acid levels and risk for vascular diseases in patients with metabolic syndrome. J Rheumatol. 2007; 34:1882–7.
20. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011; 63:3136–41.

21. Mozumdar A, Liguori G. Persistent increase of prevalence of metabolic syndrome among U.S. adults: NHANES III to NHANES 1999-2006. Diabetes Care. 2011; 34:216–9.

22. Lim S, Shin H, Song JH, Kwak SH, Kang SM, Won Yoon J, et al. Increasing prevalence of metabolic syndrome in Korea: the Korean National Health and Nutrition Examination Survey for 1998-2007. Diabetes Care. 2011; 34:1323–8.
23. Rho YH, Choi SJ, Lee YH, Ji JD, Choi KM, Baik SH, et al. Prevalence of the metabolic syndrome in patients with gout. J Korean Rheum Assoc. 2004; 11:349–57.
24. Sun HL, Pei D, Lue KH, Chen YL. Uric acid levels can predict metabolic syndrome and hypertension in adolescents: a 10-year longitudinal study. PLoS One. 2015; 10:e0143786.

25. Yokoi Y, Kondo T, Okumura N, Shimokata K, Osugi S, Maeda K, et al. Serum uric acid as a predictor of future hypertension: Stratified analysis based on body mass index and age. Prev Med. 2016; 90:201–6.

26. Wang J, Qin T, Chen J, Li Y, Wang L, Huang H, et al. Hyperuricemia and risk of incident hypertension: a systematic review and meta-analysis of observational studies. PLoS One. 2014; 9:e114259.

27. Leiba A, Vinker S, Dinour D, Holtzman EJ, Shani M. Uric acid levels within the normal range predict increased risk of hypertension: a cohort study. J Am Soc Hypertens. 2015; 9:600–9.

28. Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001; 38:1101–6.

29. Mazzali M, Kanellis J, Han L, Feng L, Xia YY, Chen Q, et al. Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am J Physiol Renal Physiol. 2002; 282:F991–7.
30. Xu W, Huang Y, Li L, Sun Z, Shen Y, Xing J, et al. Hyperuricemia induces hypertension through activation of renal epithelial sodium channel (ENaC). Metabolism. 2016; 65:73–83.

31. Mallamaci F, Testa A, Leonardis D, Tripepi R, Pisano A, Spoto B, et al. A polymorphism in the major gene regulating serum uric acid associates with clinic SBP and the white-coat effect in a family-based study. J Hypertens. 2014; 32:1621–8.

32. Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA. 2008; 300:924–32.
33. Beattie CJ, Fulton RL, Higgins P, Padmanabhan S, McCallum L, Walters MR, et al. Allopurinol initiation and change in blood pressure in older adults with hypertension. Hypertension. 2014; 64:1102–7.

34. Kohagura K, Tana T, Higa A, Yamazato M, Ishida A, Nagahama K, et al. Effects of xanthine oxidase inhibitors on renal function and blood pressure in hypertensive patients with hyperuricemia. Hypertens Res. 2016; 39:593–7.

36. Abbott RD, Brand FN, Kannel WB, Castelli WP. Gout and coronary heart disease: the Framingham study. J Clin Epidemiol. 1988; 41:237–42.

37. Krishnan E, Pandya BJ, Chung L, Dabbous O. Hyperuricemia and the risk for subclinical coronary atherosclerosis– data from a prospective observational cohort study. Arthritis Res Ther. 2011; 13:R66.
38. Stack AG, Hanley A, Casserly LF, Cronin CJ, Abdalla AA, Kiernan TJ, et al. Independent and conjoint associations of gout and hyperuricaemia with total and cardiovascular mortality. QJM. 2013; 106:647–58.

39. Erdogan D, Gullu H, Caliskan M, Yildirim E, Bilgi M, Ulus T, et al. Relationship of serum uric acid to measures of endothelial function and atherosclerosis in healthy adults. Int J Clin Pract. 2005; 59:1276–82.

40. Kato M, Hisatome I, Tomikura Y, Kotani K, Kinugawa T, Ogino K, et al. Status of endothelial dependent vasodilation in patients with hyperuricemia. Am J Cardiol. 2005; 96:1576–8.

41. Liu P, Wang H, Zhang F, Chen Y, Wang D, Wang Y. The effects of allopurinol on the carotid intima-media thickness in patients with type 2 diabetes and asymptomatic hyperuricemia: a three-year randomized parallel-controlled study. Intern Med. 2015; 54:2129–37.

42. Ogino K, Kato M, Furuse Y, Kinugasa Y, Ishida K, Osaki S, et al. Uric acid-lowering treatment with benzbromarone in patients with heart failure: a double-blind placebo-controlled crossover preliminary study. Circ Heart Fail. 2010; 3:73–81.

43. Xu X, Hu X, Lu Z, Zhang P, Zhao L, Wessale JL, et al. Xanthine oxidase inhibition with febuxostat attenuates systolic overload-induced left ventricular hypertrophy and dysfunction in mice. J Card Fail. 2008; 14:746–53.

44. Givertz MM, Anstrom KJ, Redfield MM, Deswal A, Haddad H, Butler J, et al. Effects of xanthine oxidase inhibition in hyperuricemic heart failure patients: the xanthine oxidase inhibition for hyperuricemic heart failure patients (EXACT-HF) study. Circulation. 2015; 131:1763–71.
45. Kanellis J, Watanabe S, Li JH, Kang DH, Li P, Nakagawa T, et al. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mi-togen-activated protein kinase and cyclooxygenase-2. Hypertension. 2003; 41:1287–93.

46. Kırça M, Oğuz N, Çetin A, Uzuner F, Yeşilkaya A. Uric acid stimulates proliferative pathways in vascular smooth muscle cells through the activation of p38 MAPK, p44/42 MAPK and PDGFRβ. J Recept Signal Transduct Res. 2017; 37:167–73.

47. Yu MA, Sánchez-Lozada LG, Johnson RJ, Kang DH. Oxidative stress with an activation of the renin-angiotensin system in human vascular endothelial cells as a novel mechanism of uric acid-induced endothelial dysfunction. J Hypertens. 2010; 28:1234–42.

48. Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988; 37:1595–607.

49. Krishnan E. Inflammation, oxidative stress and lipids: the risk triad for atherosclerosis in gout. Rheumatology (Oxford). 2010; 49:1229–38.

50. Facchini F, Chen YD, Hollenbeck CB, Reaven GM. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA. 1991; 266:3008–11.

51. Muscelli E, Natali A, Bianchi S, Bigazzi R, Galvan AQ, Sironi AM, et al. Effect of insulin on renal sodium and uric acid handling in essential hypertension. Am J Hypertens. 1996; 9:746–52.

52. Quiñones Galvan A, Natali A, Baldi S, Frascerra S, Sanna G, Ciociaro D, et al. Effect of insulin on uric acid excretion in humans. Am J Physiol. 1995; 268:E1–5.
53. Doshi M, Takiue Y, Saito H, Hosoyamada M. The increased protein level of URAT1 was observed in obesity/metabolic syndrome model mice. Nucleosides Nucleotides Nucleic Acids. 2011; 30:1290–4.

54. Carnethon MR, Fortmann SP, Palaniappan L, Duncan BB, Schmidt MI, Chambless LE. Risk factors for progression to incident hyperinsulinemia: the Atherosclerosis Risk in Communities Study, 1987-1998. Am J Epidemiol. 2003; 158:1058–67.

55. Choi HK, De Vera MA, Krishnan E. Gout and the risk of type 2 diabetes among men with a high cardiovascular risk profile. Rheumatology (Oxford). 2008; 47:1567–70.

56. Baldwin W, McRae S, Marek G, Wymer D, Pannu V, Baylis C, et al. Hyperuricemia as a mediator of the proinflammatory endocrine imbalance in the adipose tissue in a murine model of the metabolic syndrome. Diabetes. 2011; 60:1258–69.

57. Cicerchi C, Li N, Kratzer J, Garcia G, Roncal-Jimenez CA, Tanabe K, et al. Uric acid-dependent inhibition of AMP kinase induces hepatic glucose production in diabetes and starvation: evolutionary implications of the uricase loss in hominids. FASEB J. 2014; 28:3339–50.

58. Van den Berghe G. Fructose: metabolism and short-term effects on carbohydrate and purine metabolic pathways. Prog Biochem Pharmacol. 1986; 21:1–32.
59. Johnson RJ, Perez-Pozo SE, Sautin YY, Manitius J, Sanchez-Lozada LG, Feig DI, et al. Hypothesis: could excessive fructose intake and uric acid cause type 2 diabetes? Endocr Rev. 2009; 30:96–116.

60. Lim JS, Mietus-Snyder M, Valente A, Schwarz JM, Lustig RH. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol. 2010; 7:251–64.

61. Bomback AS, Derebail VK, Shoham DA, Anderson CA, Steffen LM, Rosamond WD, et al. Sugar-sweetened soda consumption, hyperuricemia, and kidney disease. Kidney Int. 2010; 77:609–16.

62. White JS. Straight talk about high-fructose corn syrup: what it is and what it ain't. Am J Clin Nutr. 2008; 88:1716S–21S.

63. Choi HK, Liu S, Curhan G. Intake of purine-rich foods, protein, and dairy products and relationship to serum levels of uric acid: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2005; 52:283–9.

64. Choi JW, Ford ES, Gao X, Choi HK. Sugar-sweetened soft drinks, diet soft drinks, and serum uric acid level: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2008; 59:109–16.

65. Bae J, Chun BY, Park PS, Choi BY, Kim MK, Shin MH, et al. Higher consumption of sugar-sweetened soft drinks increases the risk of hyperuricemia in Korean population: The Korean Multi-Rural Communities Cohort Study. Semin Arthritis Rheum. 2014; 43:654–61.

66. Fox IH, Kelley WN. Studies on the mechanism of fruc-tose-induced hyperuricemia in man. Metabolism. 1972; 21:713–21.

67. Choi HK, Curhan G. Soft drinks, fructose consumption, and the risk of gout in men: prospective cohort study. BMJ. 2008; 336:309–12.

68. Choi HK, Willett W, Curhan G. Fructose-rich beverages and risk of gout in women. JAMA. 2010; 304:2270–8.

69. al-Nagdy S, Miller DS, Yudkin J. Changes in body composition and metabolism induced by sucrose in the rat. Nutr Metab. 1970; 12:193–219.

70. Sleder J, Chen YD, Cully MD, Reaven GM. Hyperinsuline-mia in fructose-induced hypertriglyceridemia in the rat. Metabolism. 1980; 29:303–5.

71. Bruckdorfer KR, Kang SS, Yudkin J. Plasma concentrations of insulin, corticosterone, lipids and sugars in rats fed on meals with glucose and fructose. Proc Nutr Soc. 1973; 32:12A–3A.
72. Nakagawa T, Hu H, Zharikov S, Tuttle KR, Short RA, Glushakova O, et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol. 2006; 290:F625–31.

73. Sánchez-Lozada LG, Tapia E, Bautista-García P, Soto V, Avila-Casado C, Vega-Campos IP, et al. Effects of febuxostat on metabolic and renal alterations in rats with fructose-induced metabolic syndrome. Am J Physiol Renal Physiol. 2008; 294:F710–8.

74. Hsu MC, Wang ME, Jiang YF, Liu HC, Chen YC, Chiu CH. Long-term feeding of high-fat plus high-fructose diet induces isolated impaired glucose tolerance and skeletal muscle insulin resistance in miniature pigs. Diabetol Metab Syndr. 2017; 9:81.

75. Cheung KJ, Tzameli I, Pissios P, Rovira I, Gavrilova O, Ohtsubo T, et al. Xanthine oxidoreductase is a regulator of adipogenesis and PPARgamma activity. Cell Metab. 2007; 5:115–28.
Go to : 
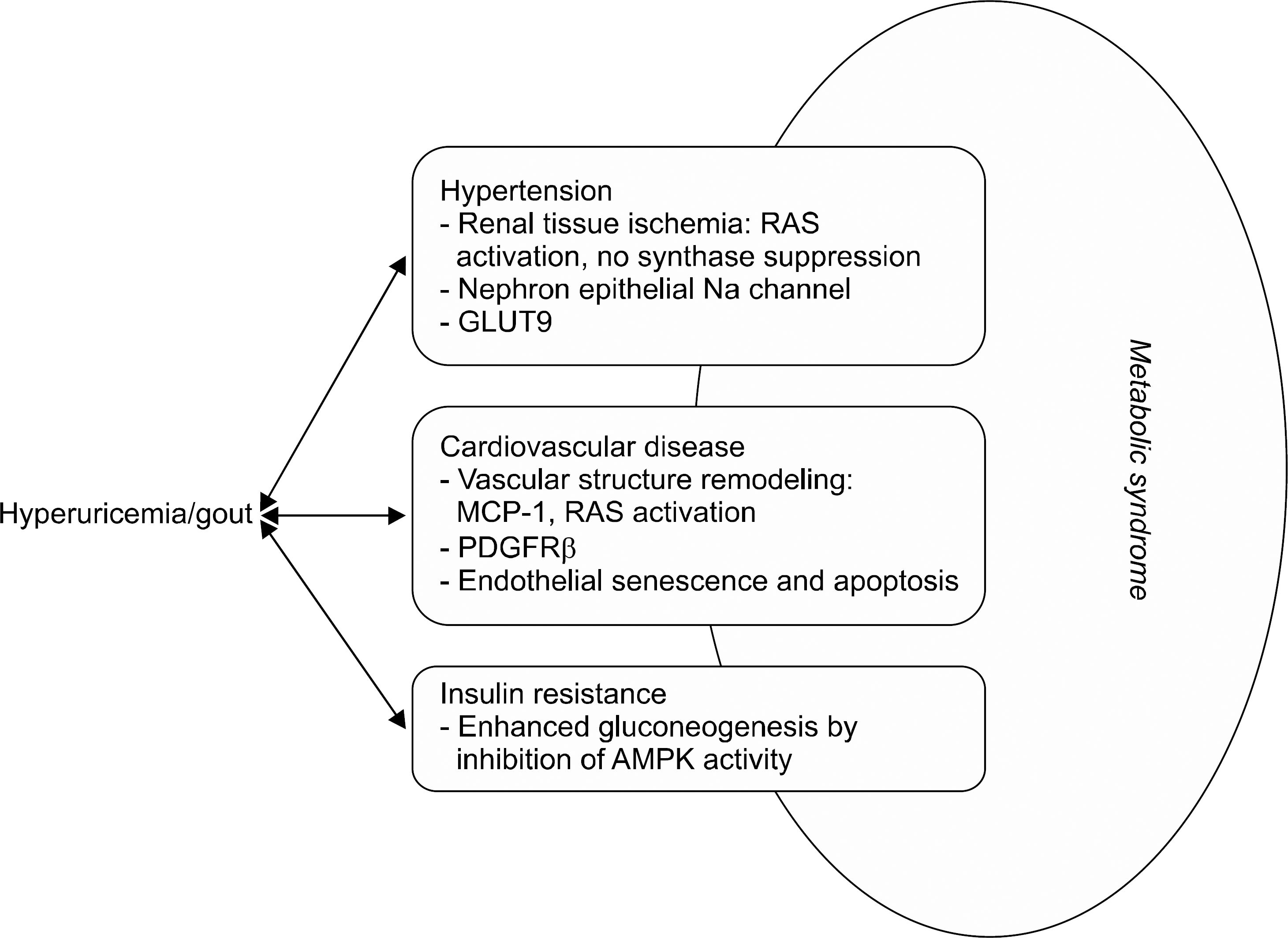 | Figure 1.Uric acid contributes to different component features of metabolic syndrome. RAS: renin-angiotensin system, NO: nitric oxide, GLUT9: glucose transporter 9, MCP-1: monocyte chemoattractant protein-1, PDGFRβ: platelet-derived growth factor receptor beta, AMPK: adenosine monophosphate kinase. |
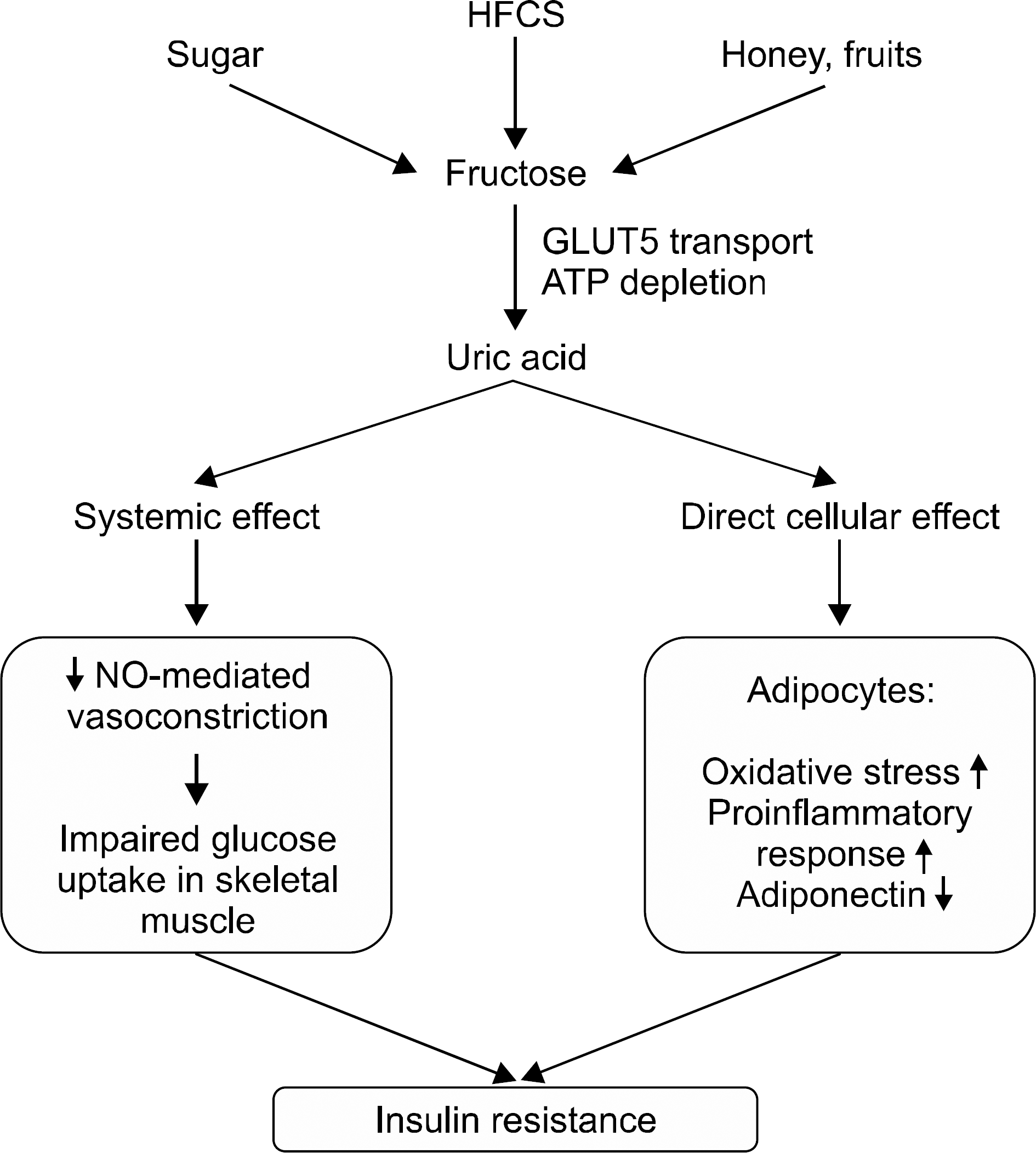 | Figure 2.Mechanisms of insulin resistance by fructose and uric acid. HFCS: high-fructose corn syrup, GLUT5: glucose transporter 5, ATP: adenosine triphosphate, NO: nitric oxide. |
Table 1.
Definitions of the metabolic syndrome: comparison of WHO, NCEP ATP III, and IDF classification criteria
WHO: World Health Organization, NCEP ATP III: National Cholesterol Education Program Adult Treatment Panel III, IDF: International Diabetes Federation, HDL: highdensity lipoprotein, DM: diabetes mellitus, IFG: impaired fasting glucose (fasting plasma glucose level, 100∼125 mg/dL), IGT: impaired glucose tolerance (2 h plasma glucose level after 75 g glucose load, 140∼199 mg/dL), IR: insulin resistance, WC: waist circumference, BMI: body mass index.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download