Abstract
Radiotherapy is used to treat not only malignant tumors but also benign inflammatory and hypertrophic diseases. Because of concerns about the potential hazards of irradiation, physicians in many countries, especially in North America, ruled radio-therapy out of medical practice for non-malignant diseases. Low-dose radiotherapy modulates the inflammatory response, providing an anti-inflammatory effect. Many researchers have reported low-dose radiotherapy efficacious for degenerative and inflammatory diseases. There are broad potential clinical indications for radiotherapy of non-malignant diseases. The general indications for radiotherapy for non-malignant disorders are acute/chronic painful degenerative diseases, such as chronic or acute painful osteoarthritic diseases of various joints; hypertrophic (hyperproliferative) disorders of soft tissues, such as early stages of Morbus Dupuytren and Ledderhose, keloids and pterygium; functional diseases, such as dysthyroid ophthalmopathy and arteriovenous malformations; and others, such as prophylaxis of heterotopic ossification. Radiotherapy for non-malignant disorders may be safely and effectively used, especially in older patients who suffered from these disorders and those who are re-luctant to use other treatment options.
Go to : 
REFERENCES
1. Leer JW, van Houtte P, Davelaar J. Indications and treatment schedules for irradiation of benign diseases: a survey. Radiother Oncol. 1998; 48:249–57.

2. Cannon B, Randolph JG, Murray JE. Malignant irradiation for benign conditions. N Engl J Med. 1959; 260:197–202.

3. Brown WM, Doll R. Mortality from cancer and other causes after radiotherapy for ankylosing spondylitis. Br Med J. 1965; 2:1327–32.

4. Mattsson A, Rudén BI, Hall P, Wilking N, Rutqvist LE. Radiation-induced breast cancer: long-term follow-up of radiation therapy for benign breast disease. J Natl Cancer Inst. 1993; 85:1679–85.

5. Reichl B, Block A, Schäfer U, Bert C, Müller R, Jung H, et al. DEGRO practical guidelines for radiotherapy of non-malignant disorders: Part I: physical principles, radiobiological mechanisms, and radiogenic risk. Strahlenther Onkol. 2015; 191:701–9.
6. Speyer CL, Ward PA. Role of endothelial chemokines and their receptors during inflammation. J Invest Surg. 2011; 24:18–27.

7. Roedel F, Kley N, Beuscher HU, Hildebrandt G, Keilholz L, Kern P, et al. Anti-inflammatory effect of low-dose X-irradiation and the involvement of a TGF-beta1-induced downregulation of leukocyte/endothelial cell adhesion. Int J Radiat Biol. 2002; 78:711–9.
8. Smith WB, Noack L, Khew-Goodall Y, Isenmann S, Vadas MA, Gamble JR. Transforming growth factor-beta 1 inhibits the production of IL-8 and the transmigration of neutrophils through activated endothelium. J Immunol. 1996; 157:360–8.
9. Rödel F, Schaller U, Schultze-Mosgau S, Beuscher HU, Keilholz L, Herrmann M, et al. The induction of TGF-be-ta(1) and NF-kappaB parallels a biphasic time course of leukocyte/endothelial cell adhesion following low-dose X-irradiation. Strahlenther Onkol. 2004; 180:194–200.
10. Prasad AV, Mohan N, Chandrasekar B, Meltz ML. Activation of nuclear factor kappa B in human lymphoblastoid cells by low-dose ionizing radiation. Radiat Res. 1994; 138:367–72.
11. Rödel F, Hantschel M, Hildebrandt G, Schultze-Mosgau S, Rödel C, Herrmann M, et al. Dose-dependent biphasic induction and transcriptional activity of nuclear factor kappa B (NF-kappaB) in EA.hy.926 endothelial cells after low-dose X-irradiation. Int J Radiat Biol. 2004; 80:115–23.
12. Jennette JC, Falk RJ. Pathogenesis of antineutrophil cytoplasmic autoantibody-mediated disease. Nat Rev Rheumatol. 2014; 10:463–73.

13. Wright HL, Moots RJ, Edwards SW. The multifactorial role of neutrophils in rheumatoid arthritis. Nat Rev Rheumatol. 2014; 10:593–601.

14. Rödel F, Hofmann D, Auer J, Keilholz L, Röllinghoff M, Sauer R, et al. The anti-inflammatory effect of low-dose radiation therapy involves a diminished CCL20 chemokine expression and granulocyte/endothelial cell adhesion. Strahlenther Onkol. 2008; 184:41–7.

15. Kern P, Keilholz L, Forster C, Seegenschmiedt MH, Sauer R, Herrmann M. In vitro apoptosis in peripheral blood mononuclear cells induced by low-dose radiotherapy displays a discontinuous dose-dependence. Int J Radiat Biol. 1999; 75:995–1003.
16. Gaipl US, Meister S, Lödermann B, Rödel F, Fietkau R, Herrmann M, et al. Activation-induced cell death and total Akt content of granulocytes show a biphasic course after low-dose radiation. Autoimmunity. 2009; 42:340–2.

17. Wunderlich R, Ernst A, Rödel F, Fietkau R, Ott O, Lauber K, et al. Low and moderate doses of ionizing radiation up to 2 Gy modulate transmigration and chemotaxis of activated macrophages, provoke an anti-inflammatory cytokine mi-lieu, but do not impact upon viability and phagocytic function. Clin Exp Immunol. 2015; 179:50–61.

18. Hildebrandt G, Jahns J, Hindemith M, Spranger S, Sack U, Kinne RW, et al. Effects of low dose radiation therapy on adjuvant induced arthritis in rats. Int J Radiat Biol. 2000; 76:1143–53.
19. Hildebrandt G, Loppnow G, Jahns J, Hindemith M, Anderegg U, Saalbach A, et al. Inhibition of the iNOS pathway in inflammatory macrophages by low-dose X-irradiation in vitro. Is there a time dependence? Strahlenther Onkol. 2003; 179:158–66.

20. Schaue D, Jahns J, Hildebrandt G, Trott KR. Radiation treatment of acute inflammation in mice. Int J Radiat Biol. 2005; 81:657–67.

21. Arenas M, Gil F, Gironella M, Hernández V, Jorcano S, Biete A, et al. Anti-inflammatory effects of low-dose radiotherapy in an experimental model of systemic inflammation in mice. Int J Radiat Oncol Biol Phys. 2006; 66:560–7.

22. Frey B, Gaipl US, Sarter K, Zaiss MM, Stillkrieg W, Rödel F, et al. Whole body low dose irradiation improves the course of beginning polyarthritis in human TNF-transgenic mice. Autoimmunity. 2009; 42:346–8.

23. Liebmann A, Hindemith M, Jahns J, Madaj-Sterba P, Weisheit S, Kamprad F, et al. Low-dose X-irradiation of adjuvantinduced arthritis in rats. Efficacy of different fractionation schedules. Strahlenther Onkol. 2004; 180:165–72.
24. Micke O, Seegenschmiedt MH. German Working Group on Radiotherapy in Germany. Consensus guidelines for radiation therapy of benign diseases: a multicenter approach in Germany. Int J Radiat Oncol Biol Phys. 2002; 52:496–513.

25. Ott OJ, Hertel S, Gaipl US, Frey B, Schmidt M, Fietkau R. Benign painful elbow syndrome. First results of a single center prospective randomized radiotherapy dose optimization trial. Strahlenther Onkol. 2012; 188:873–7.
26. Ott OJ, Hertel S, Gaipl US, Frey B, Schmidt M, Fietkau R. Benign painful shoulder syndrome: initial results of a single-center prospective randomized radiotherapy dose-opti-mization trial. Strahlenther Onkol. 2014; 188:1108–13.
27. Micke O, Seegenschmiedt MH, Adamietz IA, Kundt G, Fakhrian K, Schaefer U, et al. Low-dose radiation therapy for benign painful skeletal disorders: the typical treatment for the elderly patient? Int J Radiat Oncol Biol Phys. 2016 Dec 18; [Epub].DOI: DOI: 10.1016/j.ijrobp.2016.12.012.

28. Ott OJ, Jeremias C, Gaipl US, Frey B, Schmidt M, Fietkau R. Radiotherapy for benign achillodynia. Long-term results of the Erlangen Dose Optimization Trial. Strahlenther Onkol. 2015; 191:979–84.
29. Trott KR, Kamprad F. Radiobiological mechanisms of antiinflammatory radiotherapy. Radiother Oncol. 1999; 51:197–203.

30. Seegenschmiedt MH, Keilholz L. Epicondylopathia humeri (EPH) and peritendinitis humeroscapularis (PHS): evaluation of radiation therapy long-term results and literature review. Radiother Oncol. 1998; 47:17–28.

31. Niewald M, Fleckenstein J, Naumann S, Ruebe C. Long-term results of radiotherapy for periarthritis of the shoulder: a retrospective evaluation. Radiat Oncol. 2007; 2:34.

32. Micke O, Seegenschmiedt MH. German Cooperative Group on Radiotherapy for Benign Diseases. Radiotherapy in painful heel spurs (plantar fasciitis)–results of a national patterns of care study. Int J Radiat Oncol Biol Phys. 2004; 58:828–43.

33. Badakhshi H, Buadch V. Low dose radiotherapy for plantar fasciitis. Treatment outcome of 171 patients. Foot (Edinb). 2014; 24:172–5.

34. Hermann RM, Meyer A, Becker A, Schneider M, Reible M, Carl UM, et al. Effect of field size and length of plantar spur on treatment outcome in radiation therapy of plantar fasciitis: the bigger the better? Int J Radiat Oncol Biol Phys. 2013; 87:1122–8.

35. Mücke R, Seegenschmiedt MH, Heyd R, Schäfer U, Prott FJ, Glatzel M, et al. [Radiotherapy in painful gonarthrosis. Results of a national patterns-of-care study]. Strahlenther Onkol. 2010; 186:7–17. German.
36. Keller S, Müller K, Kortmann RD, Wolf U, Hildebrandt G, Liebmann A, et al. Efficacy of low-dose radiotherapy in painful gonarthritis: experiences from a retrospective East German bicenter study. Radiat Oncol. 2013; 8:29.

37. Ott OJ, Niewald M, Weitmann HD, Jacob I, Adamietz IA, Schaefer U, et al. DEGRO guidelines for the radiotherapy of non-malignant disorders. Part II: Painful degenerative skeletal disorders. Strahlenther Onkol. 2015; 191:1–6.
38. Minten MJ, Mahler E, den Broeder AA, Leer JW, van den Ende CH. The efficacy and safety of low-dose radiotherapy on pain and functioning in patients with osteoarthritis: a systematic review. Rheumatol Int. 2016; 36:133–42.

39. Keilholz L, Seegenschmiedt MH, Sauer R. Radiotherapy for prevention of disease progression in early-stage Dupuytren's contracture: initial and long-term results. Int J Radiat Oncol Biol Phys. 1996; 36:891–7.

40. Betz N, Ott OJ, Adamietz B, Sauer R, Fietkau R, Keilholz L. Radiotherapy in early-stage Dupuytren's contracture. Long-term results after 13 years. Strahlenther Onkol. 2010; 186:82–90.
41. Heyd R, Dorn AP, Herkströter M, Rödel C, Müller-Schimpfle M, Fraunholz I. Radiation therapy for early stages of morbus Ledderhose. Strahlenther Onkol. 2010; 186:24–9.

42. Seegenschmiedt MH, Keilholz L, Wielpütz M, Schubert C, Fehlauer F. Long-term outcome of radiotherapy for early stage Dupuytren's disease: a phase III clinical study. Eaton C, Seegenschmiedt MH, Bayat A, Gabbiani G, Werker P, Wach W, editors. Dupuytren's disease and related hyperproliferative disorders. Berlin: Springer-Verlag Berlin Heidelberg;2012. p. 349–71.

43. Seegenschmiedt MH, Micke O, Niewald M, Mücke R, Eich HT, Kriz J, et al. DEGRO guidelines for the radiotherapy of non-malignant disorders: part III: hyperproliferative disorders. Strahlenther Onkol. 2015; 191:541–8.
44. Mustoe TA, Cooter RD, Gold MH, Hobbs FD, Ramelet AA, Shakespeare PG, et al. International clinical recommendations on scar management. Plast Reconstr Surg. 2002; 110:560–71.

45. Kutzner J, Schneider L, Seegenschmiedt MH. [Radiotherapy of keloids. Patterns of care study – results]. Strahlenther Onkol. 2003; 179:54–8. German.
46. Guix B, Henríquez I, Andrés A, Finestres F, Tello JI, Martínez A. Treatment of keloids by high-dose-rate brachy-therapy: A seven-year study. Int J Radiat Oncol Biol Phys. 2001; 50:167–72.

47. Luo S, Benathan M, Raffoul W, Panizzon RG, Egloff DV. Abnormal balance between proliferation and apoptotic cell death in fibroblasts derived from keloid lesions. Plast Reconstr Surg. 2001; 107:87–96.

48. Norris JE. Superficial x-ray therapy in keloid management: a retrospective study of 24 cases and literature review. Plast Reconstr Surg. 1995; 95:1051–5.
49. Pakos EE, Ioannidis JP. Radiotherapy vs. nonsteroidal antiinflammatory drugs for the prevention of heterotopic ossification after major hip procedures: a meta-analysis of randomized trials. Int J Radiat Oncol Biol Phys. 2004; 60:888–95.

50. Reinartz G, Eich HT, Pohl F. DEGRO practical guidelines for the radiotherapy of non-malignant disorders – Part IV: Symptomatic functional disorders. Strahlenther Onkol. 2015; 191:295–302.
51. Mourits MP, van Kempen-Harteveld ML, García MB, Koppeschaar HP, Tick L, Terwee CB. Radiotherapy for Graves' orbitopathy: randomised placebo-controlled study. Lancet. 2000; 355:1505–9.

52. Schaefer U, Hesselmann S, Micke O, Schueller P, Bruns F, Palma C, et al. A long-term follow-up study after retro-orbi-tal irradiation for Graves' ophthalmopathy. Int J Radiat Oncol Biol Phys. 2002; 52:192–7.

53. Marquez SD, Lum BL, McDougall IR, Katkuri S, Levin PS, MacManus M, et al. Long-term results of irradiation for patients with progressive Graves' ophthalmopathy. Int J Radiat Oncol Biol Phys. 2001; 51:766–74.

54. Tsujino K, Hirota S, Hagiwara M, Fukada S, Takada Y, Hishikawa Y, et al. Clinical outcomes of orbital irradiation combined with or without systemic high-dose or pulsed corticosteroids for Graves' ophthalmopathy. Int J Radiat Oncol Biol Phys. 2000; 48:857–64.

55. Seegenschmiedt MH, Micke O, Muecke R. Radiotherapy for non-malignant disorders: state of the art and update of the evidence-based practice guidelines. Br J Radiol. 2015; 88:20150080.

56. Trott KR, Kamprad F. Estimation of cancer risks from radiotherapy of benign diseases. Strahlenther Onkol. 2006; 182:431–6.

57. McKeown SR, Hatfield P, Prestwich RJ, Shaffer RE, Taylor RE. Radiotherapy for benign disease; assessing the risk of radiation-induced cancer following exposure to intermediate dose radiation. Br J Radiol. 2015; 88:20150405.

58. Niewald M, Seegenschmiedt MH, Micke O, Graeber S, Muecke R, Schaefer V, et al. Randomized, multicenter trial on the effect of radiation therapy on plantar fasciitis (painful heel spur) comparing a standard dose with a very low dose: mature results after 12 months' follow-up. Int J Radiat Oncol Biol Phys. 2012; 84:e455–62.

59. Broerse JJ, Snijders-Keilholz A, Jansen JT, Zoetelief J, Klein C, Seegenschmiedt MH. Assessment of a carcinogenic risk for treatment of Graves' ophthalmopathy in dependence on age and irradiation geometry. Radiother Oncol. 1999; 53:205–8.

60. Morris MM, Powell SN. Irradiation in the setting of collagen vascular disease: acute and late complications. J Clin Oncol. 1997; 15:2728–35.

61. Chon BH, Loeffler JS. The effect of nonmalignant systemic disease on tolerance to radiation therapy. Oncologist. 2002; 7:136–43.

Go to : 
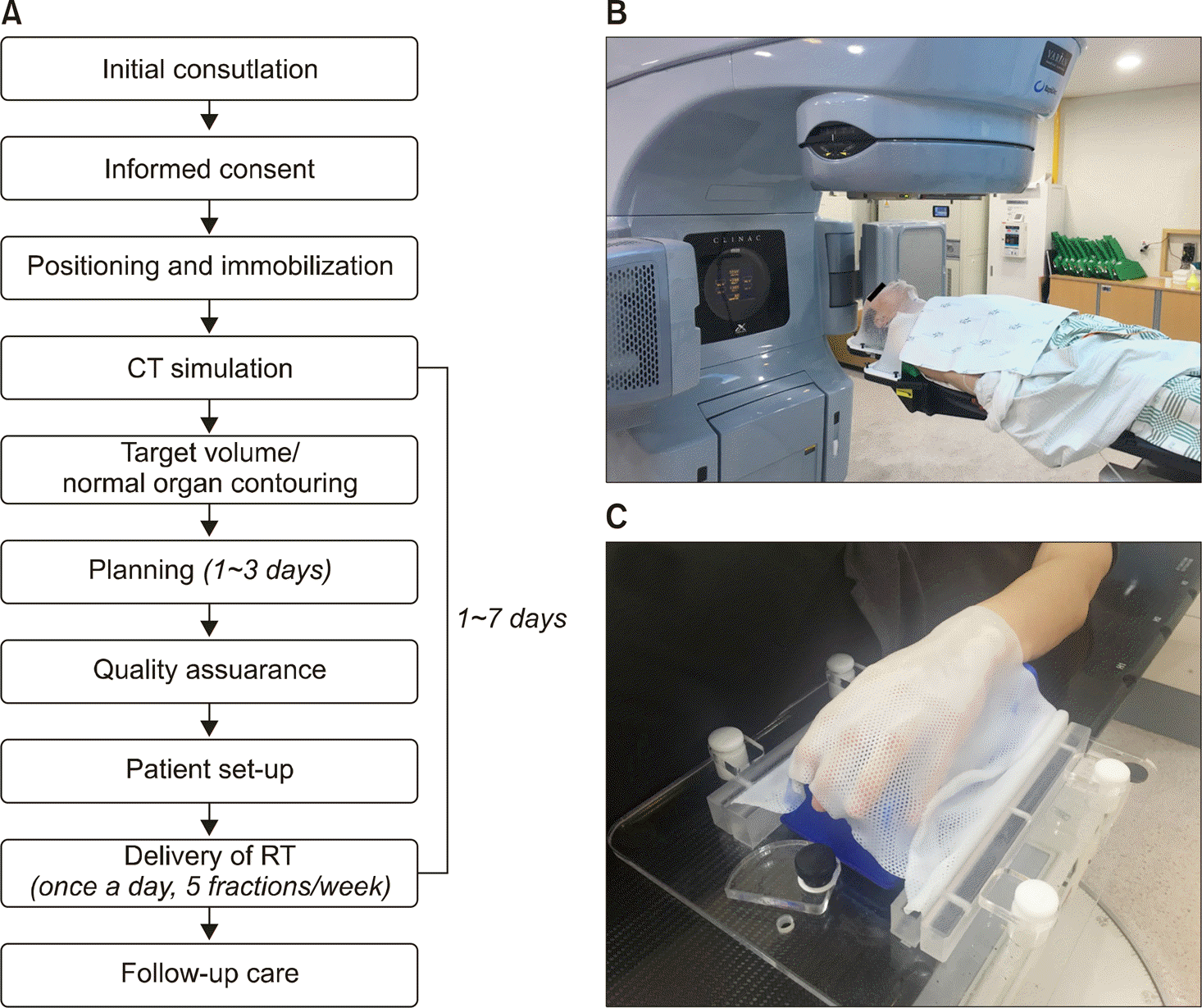 | Figure 1.(A) The process of radiotherapy planning and delivery. (B, C) Patients undergoing radiotherapy with an immobilization device in order to reduce the positioning errors and to prevent movement during radiation beam delivery. CT: computed tomography, RT: radio-therapy. |
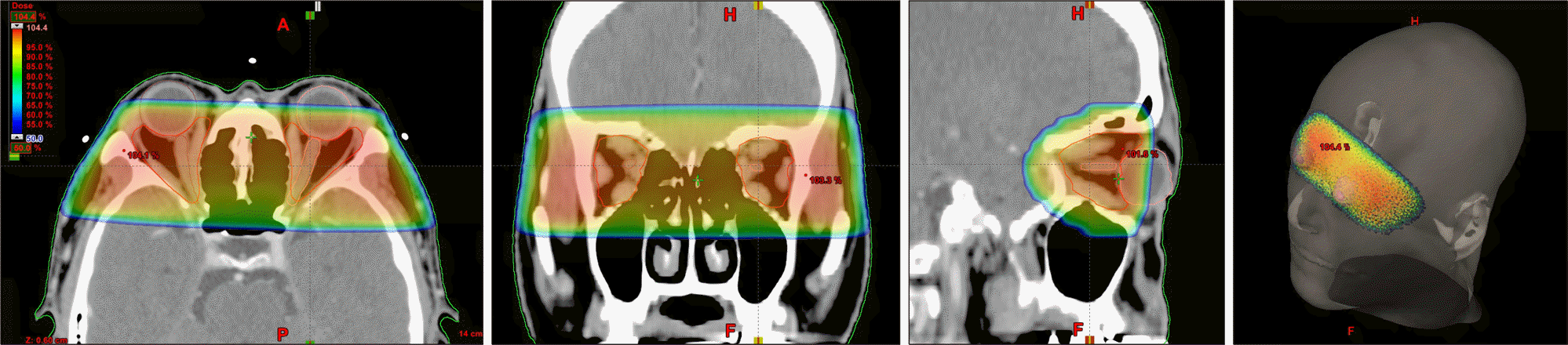 | Figure 3.The 3-dimensional conformal radiotherapy plan in a patient with dysthyroid ophthalmopathy. |
Table 1.
Summary of the studies of radiotherapy for painful degenerative diseases
| Study | Disease | Year | Patient, n | Response rate | Daily dose (Gy)×fraction | Side effect |
|---|---|---|---|---|---|---|
| Seegenschmiedt et al. [30] | Epicondylopathia humeri (EPH)/peritendinitis humeroscapularis (PHS) | 1998 | 200 | Complete response: EPH 51%, PHS 48% | 1.0×6 | No side effects |
| Partial response: EPH 20%, PHS 26% | 0.5×12 | No secondary malignancy | ||||
| Niewald et al. [31] | Periarthritis of the shoulder | 2007 | 141 | Pain relief: 69% Motility improvement: 89% | 1.0×6 (mostly) | No side effects |
| Ott et al. [25]* | Painful elbow syndrome | 2012 | 199 | Early response: 80% | 0.5×6 | NA |
| Delayed response: 91% | 1×6 | |||||
| Hermann et al. [34] | Plantar fasciitis | 2013 | 250 | Complete response: 38% | 0.5×6 | NA |
| Partial response: 32% | 1×6 | |||||
| Badakhshi et al. [33] | Plantar fasciitis | 2014 | 171 | Pain relief rate: 61.4% | 0.5×6 | NA |
| Ott et al. [26]* | Painful shoulder syndrome | 2014 | 312 | Early, delayed, and long-term response: 83%, 85%, and 82%, respectively | 0.5×6 | NA |
| 1×6 | ||||||
| Ott et al. [28]* | Achillodynia | 2015 | 112 | Early, delayed, and long-term response: 84%, 88%, and 95%, respectively | 0.5×6 | NA |
| 1×6 | ||||||
| Micke et al. [27] | Calcaneodynia, achillodynia, painful gonarthrosis, painful bursitis trochanterica | 2016 | 166 | Good response: 37.3% | 0.5∼1.0×1 | No side effects |
| 1×6 | ||||||
| 0.5×12 | ||||||
Table 2.
Specific treatment recommendations regarding radiation doses and indications by the German Working Group on Radiotherapy of Benign Diseases [24]
Table 3.
Summary of the studies of radiotherapy for Dupuytren's contracture
| Study | Disease | Year | Patient, n | Response rate | Daily dose (Gy) ×fraction | Side effect |
|---|---|---|---|---|---|---|
| Keilholz et al. [39] | Morbus Dupuytren | 1996 | 96 |
Stable: 92% Improved: 7% Progressed: 1% |
3×10 | NA |
| Betz et al. [40] | Morbus Dupuytren | 2010 | 135 | Long-term symptom relief: 66% | 3×10 |
Minor late skin toxicity: 32% No secondary malignancy |
| Heyd et al. [41] | Morbus Ledderhose | 2010 | 24 |
Complete remission of cords: 33% Reduced number: 54% Pain relief: 68.4% |
3×10 4×8 |
No RTOG grade >2 toxicity |
| Seegenschmiedt et al. [42]* | Morbus Dupuytren | 2012 | 489 |
Progression rate: 3 Gy×10, 19.5%, 3 Gy×7, 24% No RT: 62% |
3×10 3×7 No RT |
CTC grade 1: 26.5% CTC grade 2: 2.5% No secondary cancer |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download