Abstract
The rapid development of medical imaging technologies has greatly enhanced the utility of nuclear medicine imaging modalities over the last decade. Hybrid imaging technology merging computed tomography (CT) with single-photon emission computed tomography (SPECT) or positron emission tomography (PET) allows superimposing the physiologic data obtained by SPECT or PET on the detailed anatomy of CT, yielding a better understanding of the disease status and improving diagnostic performance. However, the conventional whole body bone scan and three phase bone scan still have their own distinct role as diagnostic imaging, reflecting the changes of bone metabolism in benign and malignant diseases, including rheumatic diseases. A review of each nuclear medicine imaging technique and clinical applications in various conditions of rheumatic diseases will be presented in this article.
Go to : 
REFERENCES
1. Subramanian G, McAfee JG, Bell EG, Blair RJ, O'Mara RE, Ralston PH. 99m Tc-labeled polyphosphate as a skeletal imaging agent. Radiology. 1972; 102:701–4.
2. Subramanian G. Radiopharmaceuticals for bone scanning. Collier BD, Fogelman I, Rosenthall L, editors. Skeletal nuclear medicine. St. Louis (MO): Mosby;1996. p. 9–20.
3. Freeman LM, Blaufox MD. Letter from the Editors: planar imaging in the age of SPECT. Semin Nucl Med. 2012; 42:1–2.

4. Davila D, Antoniou A, Chaudhry MA. Evaluation of osseous metastasis in bone scintigraphy. Semin Nucl Med. 2015; 45:3–15.

9. Jadvar H, Desai B, Conti PS. Sodium 18F-fluoride PET/CT of bone, joint, and other disorders. Semin Nucl Med. 2015; 45:58–65.

11. Jones AG, Francis MD, Davis MA. Bone scanning: radio-nuclidic reaction mechanisms. Semin Nucl Med. 1976; 6:3–18.

12. Elgazzar AH. Orthopedic nuclear medicine. Berlin: SpringerVerlag;2004. p. 13–31.
13. Lee WW, So Y. Skeletal system. In: Chung JK, Lee MC. Nuclear medicine. 3rd ed.Seoul: Korea Medical Book Publisher;2008. p. 509–71.
14. Eggli DF, Tulchinsky M. Normal planar bone scan. Collier BD, Fogelman I, Rosenthall L, editors. Skeletal nuclear medicine. St. Louis (MO): Mosby;1996. p. 25–6.
15. Grassi W, De Angelis R, Lamanna G, Cervini C. The clinical features of rheumatoid arthritis. Eur J Radiol. 1998; 27(Suppl 1):S18–24.

16. Ziff M. General mechanisms of inflammation in rheumatoid arthritis. Bull Schweiz Akad Med Wiss. 1979; 35:275–81.
17. Kim JY, Choi YY, Kim CW, Sung YK, Yoo DH. Bone scintigraphy in the diagnosis of rheumatoid arthritis: is there additional value of bone scintigraphy with blood pool phase over conventional bone scintigraphy? J Korean Med Sci. 2016; 31:502–9.

18. Elgazzar AH, Silberstein EB. Skeletal scintigraphy in non-neoplastic osseous disorders. Henkin RE, Bova D, Dilehay GL, Halama JR, Karesh SM, Wagner RH, editors. Nuclear medicine. 2nd ed.Philadelphia (PA): Mosby;2006. p. 1121–81.
19. Rosenthall L. Nuclear medicine techniques in arthritis. Rheum Dis Clin North Am. 1991; 17:585–97.

20. Chalmers J, Gray DH, Rush J. Observations on the induction of bone in soft tissues. J Bone Joint Surg Br. 1975; 57:36–45.

21. Craven PL, Urist MR. Osteogenesis by radioisotope labelled cell populations in implants of bone matrix under the influence of ionizing radiation. Clin Orthop Relat Res. 1971; 76:231–43.

22. Oh SN, Jee WH, Cho SM, Kim SH, Kang HS, Ryu KN, et al. Osteonecrosis in patients with systemic lupus erythematosus: MR imaging and scintigraphic evaluation. Clin Imaging. 2004; 28:305–9.
23. Ostendorf B, Mattes-György K, Reichelt DC, Blondin D, Wirrwar A, Lanzman R, et al. Early detection of bony alterations in rheumatoid and erosive arthritis of finger joints with high-resolution single photon emission computed tomography, and differentiation between them. Skeletal Radiol. 2010; 39:55–61.

24. McQueen FM. Imaging in early rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2013; 27:499–522.

25. Zilber K, Gorenberg M, Rimar D, Boulman N, Kaly L, Rozenbaum M, et al. Radionuclide methods in the diagnosis of sacroiliitis in patients with spondyloarthritis: an update. Rambam Maimonides Med J. 2016; 7:e0037.

26. Caldarella C, Isgrò MA, Treglia I, Treglia G. Is fluorine-18-fluorodeoxyglucose positron emission tomography useful in monitoring the response to treatment in patients with multiple myeloma? Int J Hematol. 2012; 96:685–91.

27. Love C, Tomas MB, Tronco GG, Palestro CJ. FDG PET of infection and inflammation. Radiographics. 2005; 25:1357–68.

28. Chaudhari AJ, Ferrero A, Godinez F, Yang K, Shelton DK, Hunter JC, et al. High-resolution (18)F-FDG PET/CT for assessing disease activity in rheumatoid and psoriatic arthritis: findings of a prospective pilot study. Br J Radiol. 2016; 89:20160138.
29. Dhawan R, Lokitz K, Lokitz S, Caldito G, Takalkar AM. FDG PET imaging of extremities in rheumatoid arthritis. J La State Med Soc. 2016; 168:156–61.
30. Suto T, Okamura K, Yonemoto Y, Okura C, Tsushima Y, Takagishi K. Prediction of large joint destruction in patients with rheumatoid arthritis using 18F-FDG PET/CT and disease activity score. Medicine (Baltimore). 2016; 95:e2841.

31. Yonemoto Y, Okamura K, Takeuchi K, Kaneko T, Kobayashi T, Okura C, et al. [18F]fluorodeoxyglucose uptake as a predictor of large joint destruction in patients with rheumatoid arthritis. Rheumatol Int. 2016; 36:109–15.

32. Wakura D, Kotani T, Takeuchi T, Komori T, Yoshida S, Makino S, et al. Differentiation between Polymyalgia Rheumatica (PMR) and elderly-onset rheumatoid arthritis using 18F-fluorodeoxyglucose positron emission tomography/computed tomography: is enthesitis a new pathological lesion in PMR? PLoS One. 2016; 11:e0158509.

33. Li Y, Schiepers C, Lake R, Dadparvar S, Berenji GR. Clinical utility of (18)F-fluoride PET/CT in benign and malignant bone diseases. Bone. 2012; 50:128–39.

Go to : 
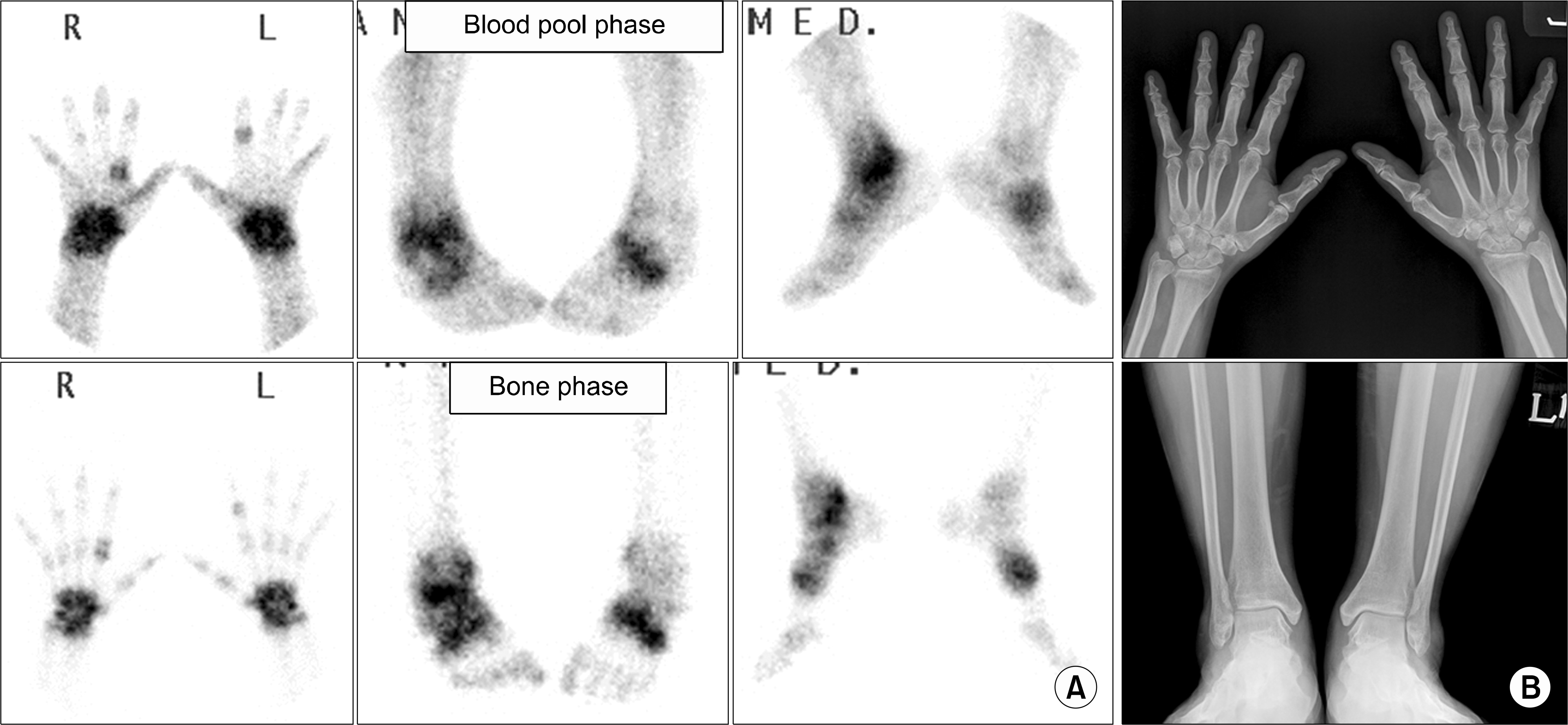 | Figure 1.Early stage of rheumatoid arthritis. (A) Blood pool phase (upper row) shows increased uptake of both hands, wrists, ankles and mid-foot, and bone phase (lower row) shows increased periarticular uptake in the same joints, suggestive of active arthritis. (B) Plain radiographs of both hands and ankles show no definite abnormal findings suggesting arthritis. Combined bone scan and radiographic findings mean increased inflammatory activity without bone change, that is suggestive of early stage of inflammatory synovitis. |
 | Figure 2.Advanced stage of rheumatoid arthritis. (A) Blood pool phase (upper row) shows subtle uptake of right 2nd, 3rd metacarpophalangeal (MCP) and left 5th MCP joints of both hands, and bone phase (lower row) shows periarticular uptake in the same joints. No abnormalities are seen in both feet. (B) Plain radiographs of both hands and feet show bony ankylosis of both wrists and right mid-foot, joint space narrowing and/or bony erosion in MCP joints of both hands. Combined bone scan and radiographic findings may be interpreted as advanced rheumatoid arthritis with decreased inflammatory activity in most joints of both hands and feet. |
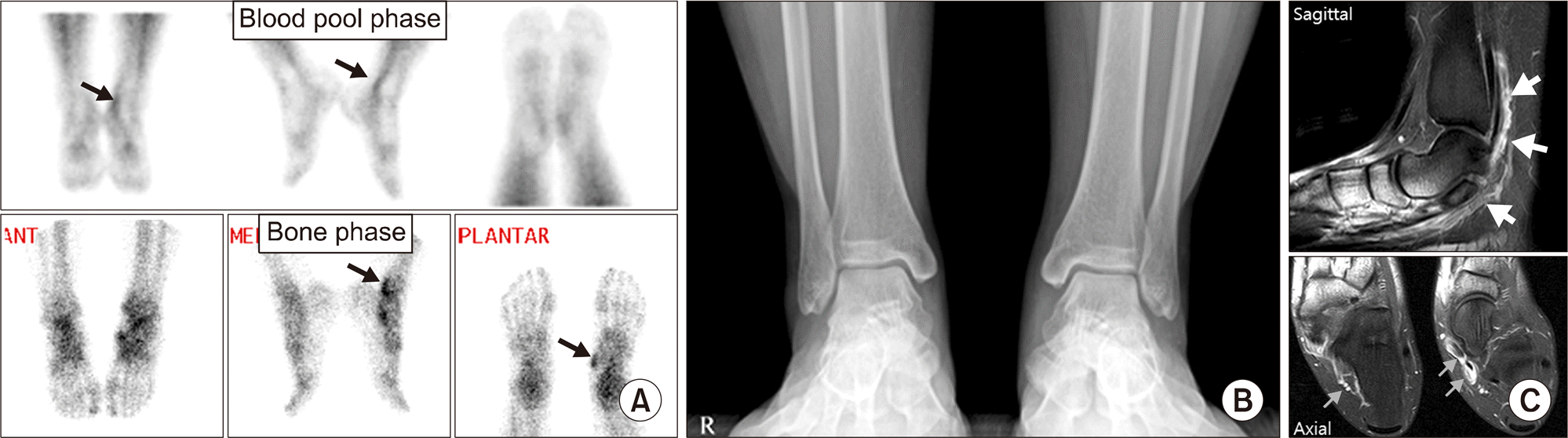 | Figure 3.Tenosynovitis of tibialis posterior tendon and flexor digitorum longus tendon. (A) Blood pool phase (upper row) shows curvilinear increased uptake in left medial ankle (arrows), and bone phase (lower row) shows focal uptake in posteroinferior aspect of medial malleolus (arrow) and navicular tuberosity (arrow), suggestive of tenosynovitis of tibialis posterior tendon and flexor digitorum longus tendon. (B) Asymmetric subtle soft tissue swelling is seen in submalleolar area of left medial ankle. (C) Magnetic resonance imaging show diffuse enhancement along the tendon sheath (arrows), suggestive of tenosynovitis. |
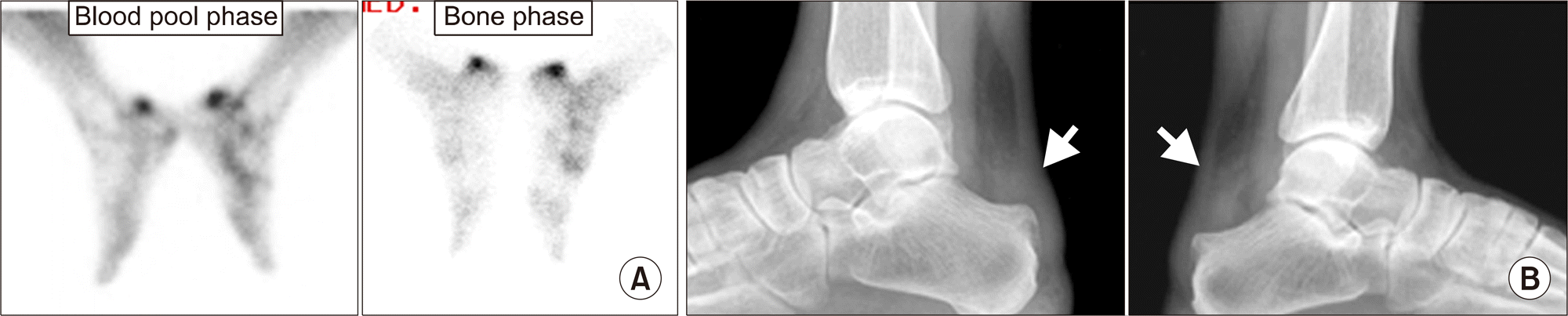 | Figure 4.Retrocalcaneal bursitis. (A) Focal increased uptake in posterior aspect of both ankles on blood pool and bone phase, suggesting possibility of retrocalcaneal bursitis or Achilles tendinitis. (B) Diffuse infiltration in retrocalcaneal fat pad (arrows) suggests bursitis of both feet. |
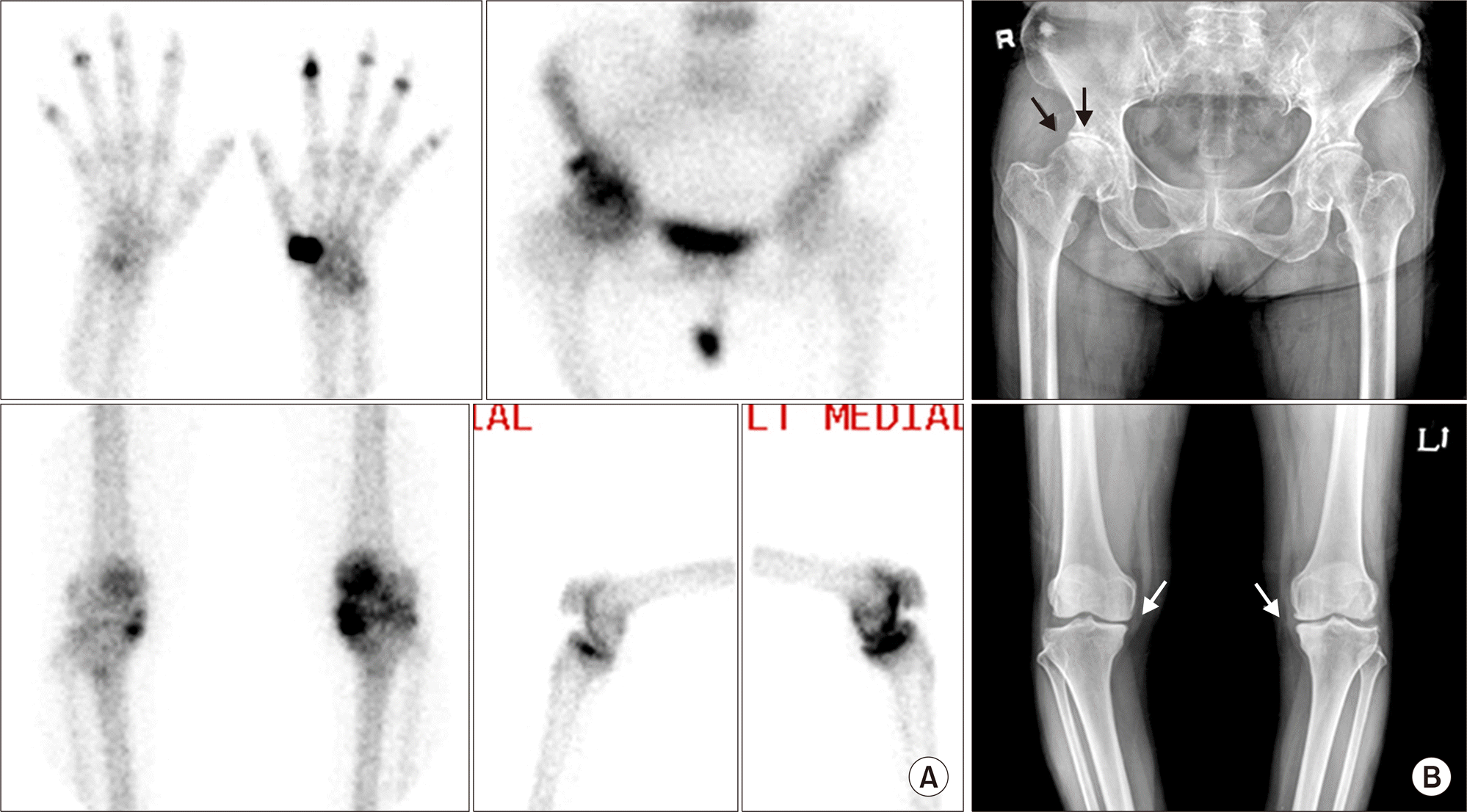 | Figure 5.Osteoarthritis. (A) Focal increased uptake lesions in left 1st carpometacarpal joint and both 2nd∼5th distal interphalangeal joints of hands, superolateral aspect of right hip joint, left patellofemoral compartment and both medial compartment of knee joints. Those are characteristic findings of osteoarthritis associated with overuse or weight bearing. (B) Plain radiographs of right hip and both knees show degenerative sclerosis, joint space narrowing, and/or spur changes (arrows). |
 | Figure 6.Multiple avascular necrosis around both knees in systemic lupus erythematosus. (A) Segmental increased uptake lesions are noted in both distal femurs and proximal tibiae (arrows), suggestive of multiple avascular necrosis. (B) Irregular calcification (arrows) and periosteal reaction (arrowheads) in both distal femurs are compatible with avascular necrosis in both distal femurs, and subtle sclerotic change is suspected in both proximal tibiae (arrows) on plain radiography. Multiple avascular necrosis may be often detected on whole body bone scan. |
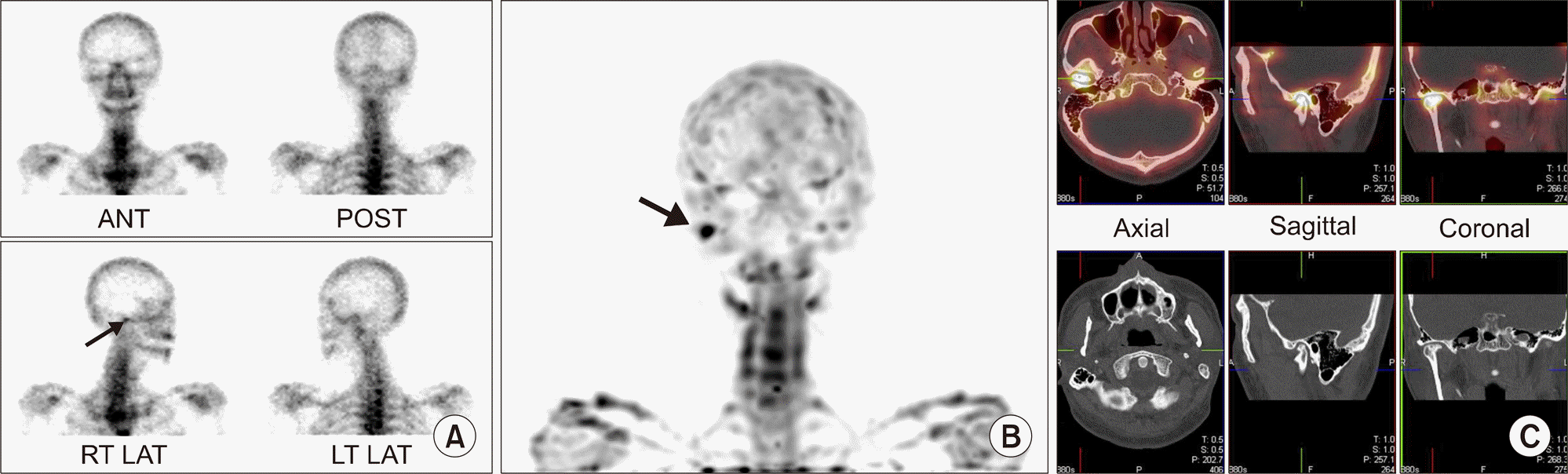 | Figure 7.Conclusive diagnosis of temporomandibular (TM) joint arthritis on single photon emission computed tomography/computed tomography (SPECT/CT). (A) Planar bone scan shows suspiciously increased uptake in right TM joint area on right anterior oblique view (arrow). (B) Maximum projection image reconstructed from SPECT image shows focal intense uptake in right TM joint (arrow). (C) Focal increased uptake in right TM joint area is matched with joint space narrowing and cortical erosion on CT image. |
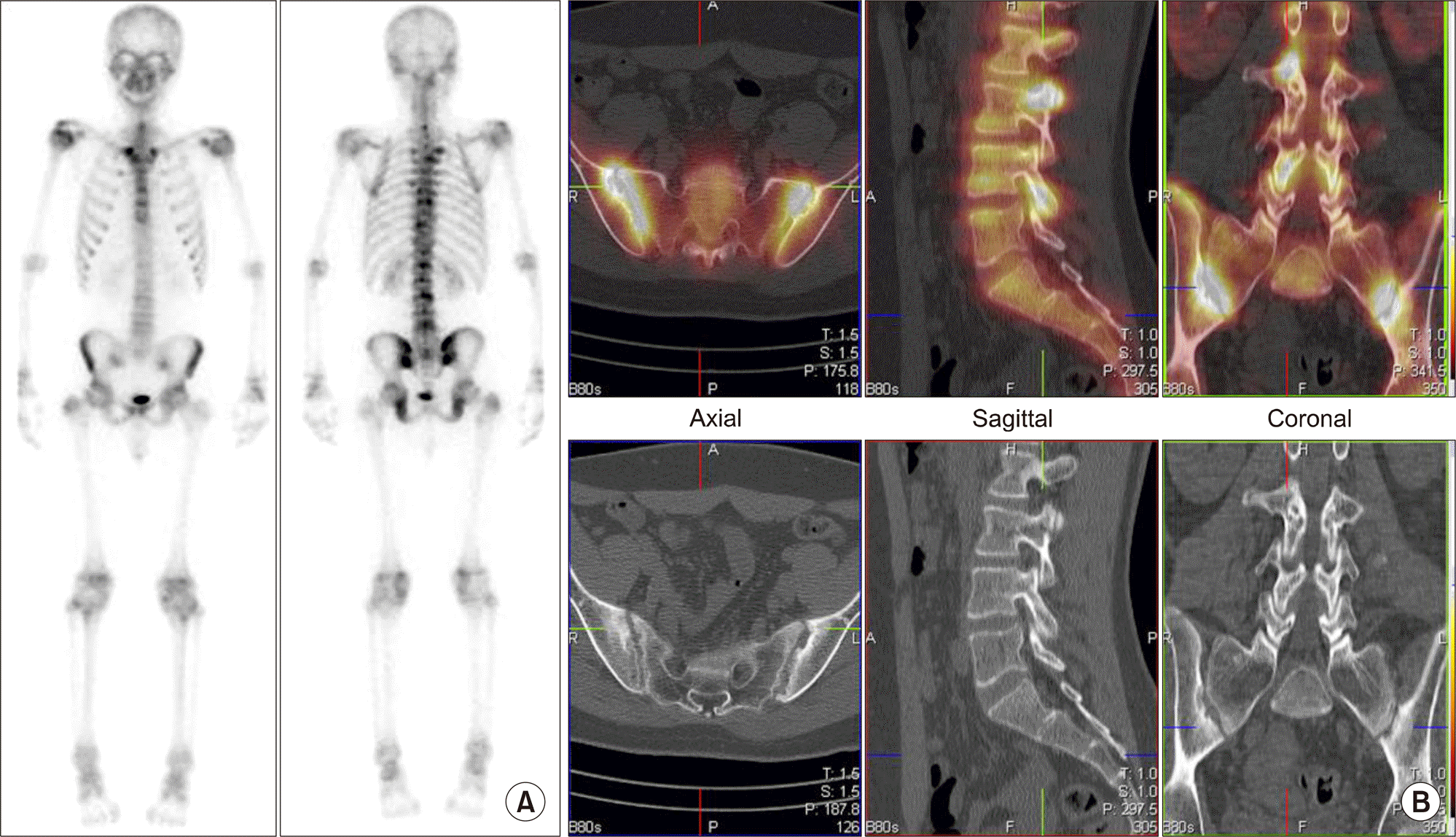 | Figure 8.Early detection of facet joint involvement in ankylosing spondylitis on single photon emission computed tomography/computed tomography. (A) There are uneven increased uptake lesions in T-L-spine on posterior whole body bone image. Lower portion of both sacroiliac (SI) joints also show mildly increased uptake. (B) Increased uptake in both SI joints, matched with joint space widening, erosion and sclerotic changes are suggestive of bilateral sacroiliitis on axial image. Focal increased uptake in L-spine are located in facet joints, but without definite anatomic change on sagittal and coronal images. |
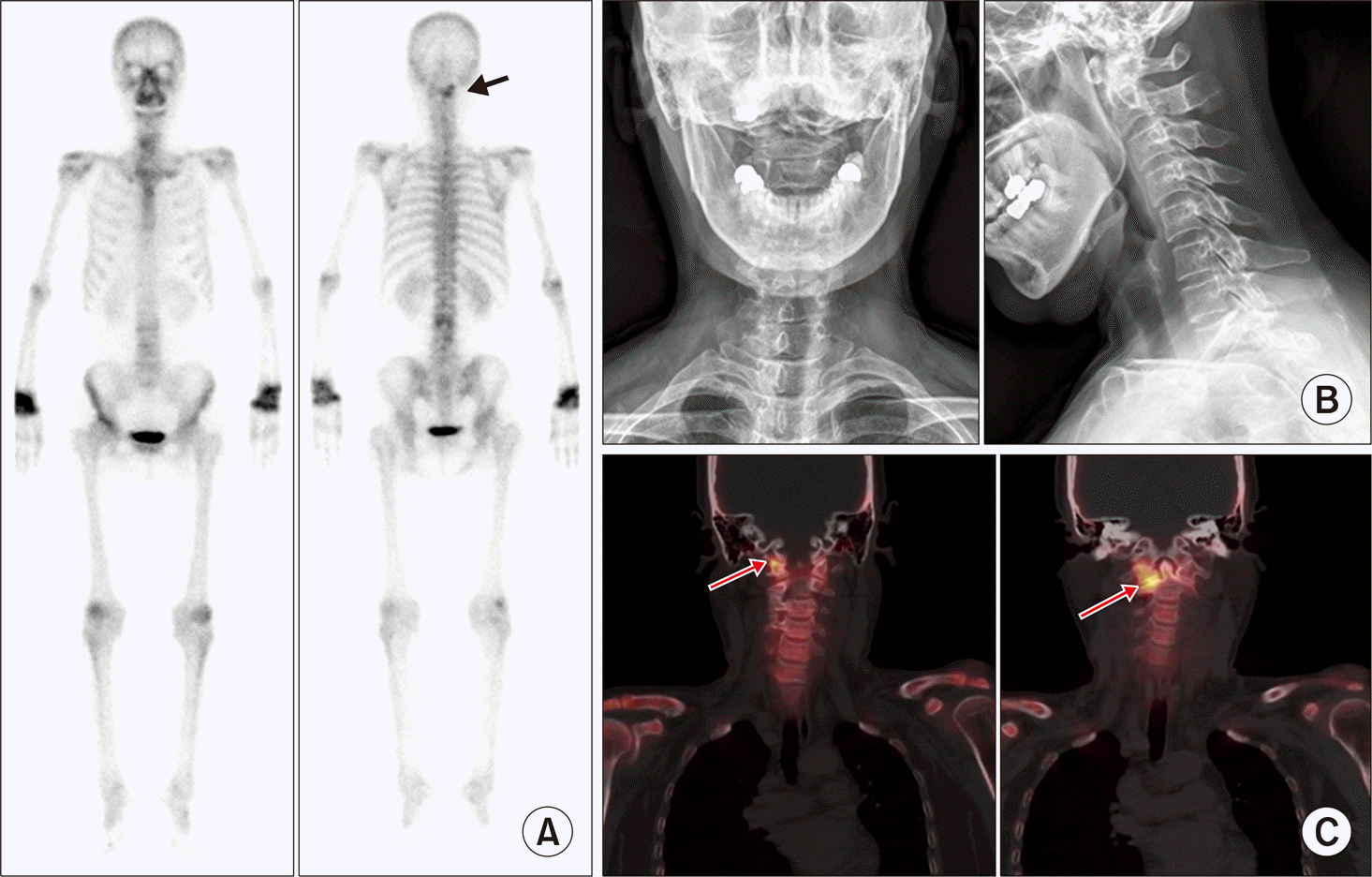 | Figure 9.Early detection of C-spine involvement of rheumatoid arthritis on single photon emission computed tomography/computed tomography. (A) Planar whole body bone scan image shows arthritis involvement of both wrists and another focal uptake lesion in right side of upper C-spine (arrow). (B) No definite abnormal findings but straightening of C-spine curvature was reported on plain radiograph. (C) Focal uptake lesion is localized in right skull base-C1 right lateral facet and C1-C2 right lateral facet (arrows), suggestive of arthritis involvement. |
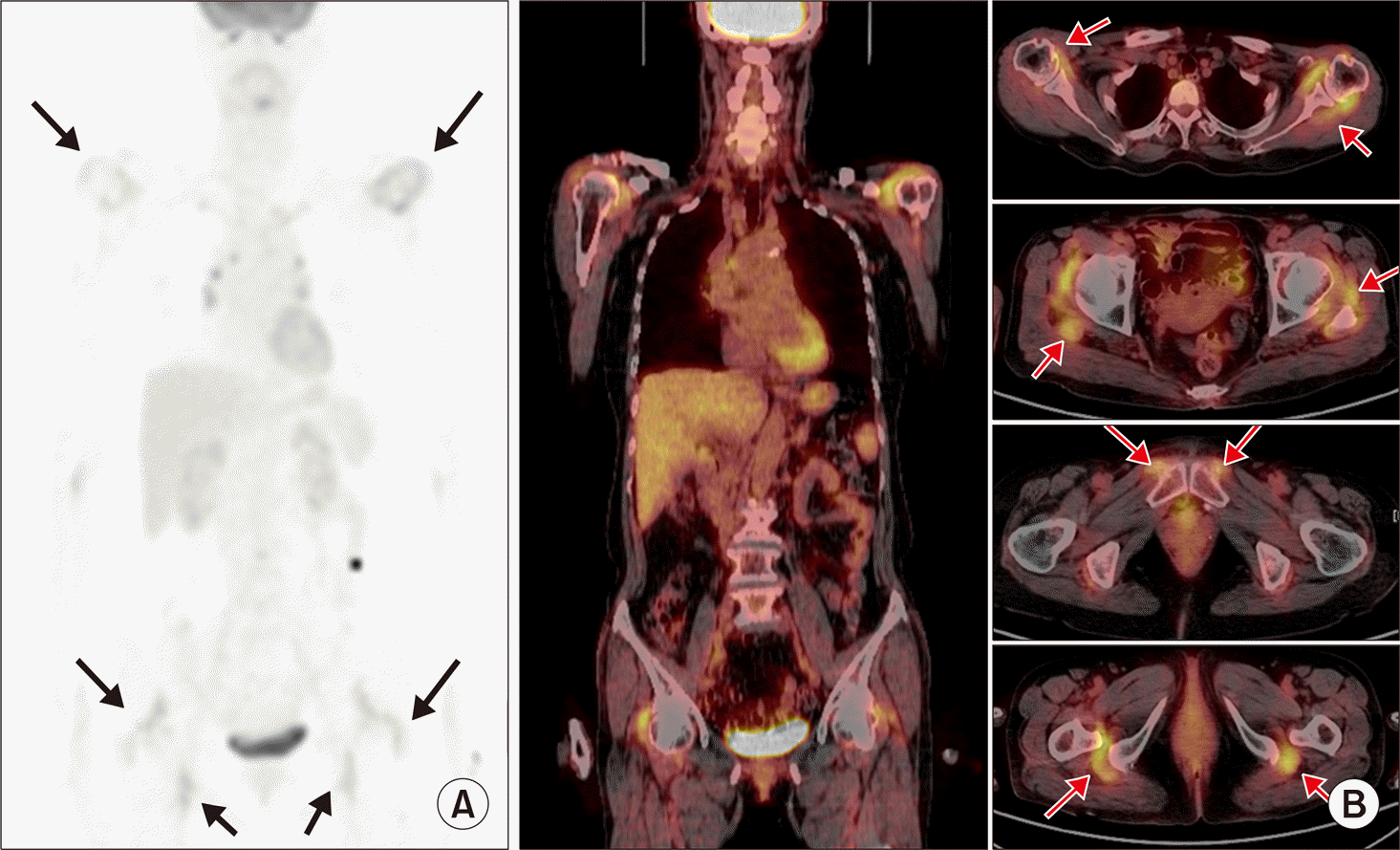 | Figure 10.Characteristic entheses inflammation of polymyalgia rheumatica (PMR) detected on F-18 fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT). (A) Increased FDG uptake around both shoulders and both proximal femurs (arrows) are noted on maximum intensity projection image, suggestive of active inflammatory process. (B) Coronal and axial PET/CT images show focal increased uptake mainly in entheses (arrows), around both shoulders, hips, pubic symphysis, and ischial tuberosity, which is characteristic areas of inflammation in PMR. |
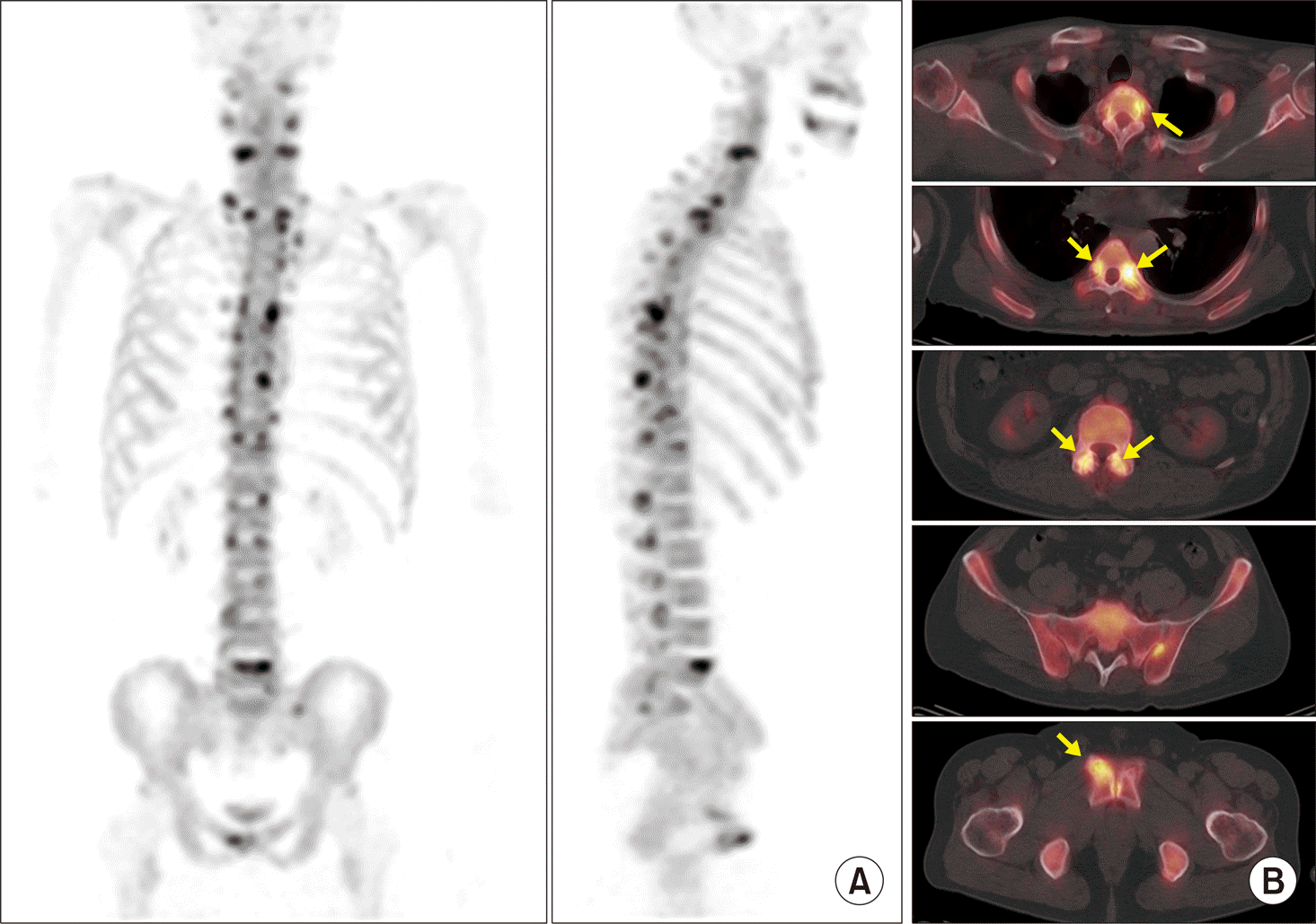 | Figure 11.Early arthritis involvement of multiple facet joints in ankylosing spondylitis detected on F-18 NaF positron emission tomography/computed tomography (PET/CT). (A) Multifocal increased uptake lesions are located mainly in posterior elements of C-T-L-spine on maximum intensity projections (MIP) images of PET/CT. Both sacroiliac (SI) joints are unremarkable.(B) Focal uptake lesions detected on MIP images are located in costovertebral junctions, facet joints, and enthesis of right anterior pubic body on axial PET/CT images (arrows). Both SI joints are not involved. |
Table 1.
Manifestations of pathologic changes of synovial joints in rheumatoid arthritis on plain radiography and bone scan with blood pool phase (BSBP)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download