Abstract
Behçet's disease (BD) is a systemic vasculitis commonly accompanied by recurrent mucosal ulceration and other systemic manifestations, but rarely by myositis. Focal eosinophilic myositis is the most limited idiopathic eosinophilic myopathy characterized by peripheral blood eosinophilia and/or eosinophilic muscle infiltration. Clinical manifestations include myalgia, muscle weakness, and cutaneous lesions, such as subcutaneous induration and erythema. Given that BD can mimic deep vein thrombosis or pseudotumor, muscle biopsy should be performed to enhance the accuracy of diagnosis. Microscopic examination reveals extensive infiltration of eosinophils and mononuclear cells into muscle, myofiber necrosis, and regeneration. To the best of our knowledge, there have not been any published reports on MEDLINE regarding focal eosinophilic myositis associated with BD. Here, we presented a case of focal eosinophilic myositis associated with intestinal BD in a 23-year-old man who suffered from a large ulcer in the terminal ileum.
Behçet's disease (BD) is a systemic vasculitis, characterized by vascular injury, hyperfunction of neutrophils, and autoimmune responses and clinically often presented with mucous membrane ulceration, skin lesions and ocular symptoms [12]. Gastrointestinal (GI) involvement in BD is particularly important as it is one of serious and difficult to treat; therefore, BD is designated “intestinal BD,” if a typical oval-shaped large ulcer in the terminal ileum or ulcerations in the GI tract are objectively documented [23]. Musculoskeletal involvement is one of the most frequent findings in BD, of which arthritis and arthralgia are the most common findings [14]. Myositis is rare manifestation in BD and is usually mild, short-lasting, and more localized than generalized form [5]. Histologic examination reveals a predominant neutrophil infiltration associated with focal necrosis and perivasculitis, suggesting vasculitis participates in the pathogenesis of myositis [16].
Eosinophilic infiltration into skeletal muscle, although rare, has been described in a diverse group of conditions, including drugs such as L-tryptophan, D-penicillamine and ethanol, parasitic infection, autoimmune diseases such as rheumatoid arthritis, myasthenia gravis and eosinophilic granulomatosis with polyangiitis, eosinophilia-myalgia syndrome, and idiopathic eosinophilic myopathy [78]. Although pathogenesis of eosinophilic myopathies is incompletely understood, it is suggested that interleukin- 5 is stimulated by a precipitating factor, and the inflammatory mediators by eosinophil including cationic protein, eosinophilic major basic protein, and enzymes, increase in the target tissues and blood [8]. Idiopathic eosinophilic myopathy is a rare, clinically and pathologically heterogeneous disease, characterized by the presence of peripheral and/or muscle eosinophilia [8]. Clinical manifestations include myalgia, muscle weakness, edematous changes of extremities, arthralgia/arthritis, and skin lesions. Cutaneous manifestations include deep subcutaneous induration, erythema, urticarial or angioedematous plaques, and erythematous papular lesions, in which deep subcutaneous induration is the most common finding [910]. Biopsy of cutaneous lesions demonstrates scanty-to-moderate perivascular mononuclear infiltrates with a considerable variability in the number of admixed eosinophils, ranging from lymphocytic vasculitis devoid of eosinophils to eosinophilic cellulitis-like lesions [9]. Most, if not all, of the patients represent peripheral or tissue eosinophilia, and elevated muscle enzymes [10].
Although connective tissue disorders may be related with eosinophilic myopathy [8] and the localized form of myopathy can be presented as the musculoskeletal manifestation of BD [45], there are no published case reports that the localized form of myopathy in patients with BD was identified as focal eosinophilic myositis when searched on MEDLINE. Herein, we present a case of focal eosinophilic myositis associated with intestinal BD in a 23-year-old man who suffered from a large ulcer in the terminal ileum.
A 23-year old man who had a history of recurrent oral ulcerations and pseudofolliculitis was admitted to our hospital with abdominal pain and a mass in the right upper arm. Six months ago, he began to have recurrent right lower abdominal pain, several subcutaneous nodules, and, at the same time, a persistent painful firm mass appeared on the right upper arm. He had no past history of drug medication or allergic diseases. The abdominal examination revealed tenderness in right lower quadrant. There were about a 3 cm-sized firm, nonmovable mass covered by normal skin at the back of right upper arm and several subcutaneous induration with tenderness of the left flank and the right lower leg. In manual muscle test, there was no evidence of muscle weakness of both upper and lower extremities. Pathergy test was positive.
Laboratory examination revealed a white blood cell count of 9,890/µL (eosinophil count 380/µL, 3.8%), erythrocyte sedimentation rate of 40 mm/h (normal, 0~10), C-reactive protein of 2.13 mg/dL (0~0.5), creatine phosphokinase of 33 µL (39~308), lactate dehydrogenase of 392 µL (0~250), myoglobin of 18 ng/mL (14~106), and aldolase of 15.4 µL (0~7.6). Results of immunological analyses were as follows: immunoglobulin (Ig) G 1,643 mg/dL (700~1,600), IgA 237 mg/dL (70~400), IgM 154 mg/dL (94~230), C3 132.3 mg/dL (90~180), C4 34.9 mg/dL (10~40), antinuclear antibody negative, rheumatoid factor negative, and anti-neutrophil cytoplasmic antibody negative.
Chest X-ray, electrocardiogram, and echocardiogram findings were all nonspecific. The patient complained of persistent abdominal pain and underwent abdominal computed tomography (CT) and colonoscopy. Abdominal CT showed a wall thickening at the terminal ileum (Figure 1A), and colonoscopy revealed a huge, round well-demarcated ulcer with central yellowish exudate at the terminal ileum (Figure 1B). The mucosal biopsies of intestinal lesions showed chronic inflammation without granuloma. Magnetic resonance image (MRI) study of the right upper arm demonstrated a focal mass lesion measuring 3.0 cm (length)×2.0 cm (width) in the right triceps muscle with a heterogeneous hyper-intense signal on T2-weighted image (Figure 2A). On a gadolinium enhancement T1-weighted image of MRI, the lesions appeared to be a well-defined rim of contrast enhancement and a hypointense central area (Figure 2B). Muscle biopsy, performed to determine the exact pathologic findings of triceps muscle, showed that myofibers became severe atrophic and were replaced by inflammatory cell infiltration with predominant eosinophils, and focal central necrosis without perivasculitis, which were consistent with focal eosinophilic myositis (Figure 3).
The patient was diagnosed as focal eosinophilic myositis associated with intestinal BD based on terminal ileal ulcer, recurrent oral ulcers, skin manifestation, and positive pathergy test. High dose prednisone at a daily dose of 1 mg/kg was administrated due to intestinal BD and focal eosinophilic myositis, and azathioprine was added for the treatment of intestinal BD. After two weeks' treatment, the size of mass lesion in the triceps muscle and several subcutaneous indurations were dramatically decreased. In a follow-up MRI taken two weeks later, the size of mass in the triceps muscle decreased considerably to 1.5 cm (length)×0.5 cm (width), although there was a slightly hypersignal intensity on T2-weighted image around the triceps muscle (Figure 4).
This is an extremely rare case of focal eosinophilic myositis associated with intestinal BD concurrently. BD is a chronic, relapsing, and debilitating systemic vasculitis of unknown etiology, which affects both arteries and veins of all sizes, thus causing a diverse spectrum of organ involvement from head to foot that can emerge at any point in time [12]. Because BD does not have any pathognomonic symptoms or laboratory findings, a diagnosis of BD is made using clinical criteria established by the International Study Group (ISG) criteria for BD, which use five items including recurrent oral and genital ulcerations, ocular lesions, skin manifestations and positive pathergy test [1]. Although GI involvement is not included in ISG criteria for BD, it shows the highest prevalence rates in the Far-Eastern countries, such as Korea and Japan [211]. Typical endoscopic findings of intestinal lesions in intestinal BD are single or a few large ulcers in the ileocecal area, which may be round or oval-shaped, deep ulcers with discrete and elevated margin [3]. Our patient, who fulfilled the ISG criteria for BD, complained of abdominal pain and was diagnosed as intestinal BD based on a large, deep ulcer with mucosal swelling in the terminal ileum on the colonoscopy and abdominal CT.
Idiopathic eosinophilic myopathy is classified into three subtypes including focal eosinophilic myositis, eosinophilic polymyositis, and eosinophilic perimyositis, in which focal eosinophilic myositis is the most limited form and has several distinguishable features [812]. Focal eosinophilic myositis usually does not present with other internal organ involvement or systemic manifestations except skin lesions and may resolve spontaneously. Muscular involvements are usually focal and circumscribed to the lower legs and thus often mistaken for deep vein thrombosis or pseudotumor [8], although involvement of other muscles such as deltoid [13] and sternocleidomastoid muscles [14] was also reported. The most frequent skin lesions are subcutaneous induration and erythema [9]. Even though MRI is the primary imaging modality for evaluating soft tissue masses of the musculoskeletal system, its findings of focal eosinophilic myositis are nonspecific, in which high signal intensity is found in the muscle tissue on T2-weighted images [1015]. Muscle biopsy is considered to be important for diagnosis, revealing extensive infiltration of eosinophils in the perimysial and endomysial tissues and within muscle fibers, associated with necrosis [781214]. Focal aggregates of lymphocytes and plasma cells and degenerative changes of muscle fibers are also observed. However, there is no perivascular infiltration [12]. Our patient, presented with a soft tissue mass with tenderness on the triceps muscle and several subcutaneous indurative lesions, did not have blood eosinophilia, elevated muscle enzymes, or the history of drug and allergic diseases. Muscle biopsy showed massive muscular infiltration of eosinophils and mononuclear cells, severe fibrous change, and focal necrosis, which was consistent with pathologic findings of focal eosinophilic myositis. In this case, skin biopsy was not performed.
Recently, a Spanish group [8] proposed a set of diagnostic criteria in which clinical, pathological and radiological findings were taken into consideration. Major criteria of the proposed criteria are as follows: 1) pain and swelling of calf (other muscles can be affected) and 2) deep mononuclear cell infiltration (eosinophilic or not), with muscle fiber invasion and necrosis on muscle biopsy. Minor criteria are as follows: 1) elevated serum levels of creatine kinase and aldolase, 2) MRI or electromyographic evidence of focal myositis, 3) absence of systemic illness and 4) eosinophilia (>0.5×109/L). Diagnosis requires presence of two major criteria or one major criterion and all three minor criteria, and deep vein thrombosis, cellulitis, and parasitic infections must be excluded. Our patient was diagnosed with the focal eosinophilic myositis based on focal painful myopathy on the upper extremity, pathologic findings including deep muscular infiltration of mononuclear cells and eosinophils with muscle fiber invasion and necrosis, and evidence of myositis on MRI.
Distinguishing between focal eosinophilic myositis and localized myopathy of BD in this case was a tough problem because our patient was diagnosed with BD and there are no published reports about focal eosinophilic myositis associated with BD when searched on MEDLINE. We made the diagnosis of focal eosinophilic myositis in this case for the following reasons. First, our patient satisfied new proposed diagnostic criteria for focal eosinophilic myositis. Second, main infiltrative cells in muscular lesions were eosinophils and mononuclear cells, not neutrophils, which was consistent with pathologic findings of focal eosinophilic myositis. Third, we did not find any perivasculitic or vasculitic findings on the muscular pathology, although there was a possibility that we could not detect these lesions because of intense infiltration of inflammatory cells and tissue necrosis. Last, in addition to myositis, our patient had several subcutaneous indurative lesions, which were one of cutaneous symptoms of idiopathic eosinophilic myositis rather than cutaneous manifestations of BD.
Focal eosinophilic myositis rapidly responds to corticosteroid therapy, although many cases run a benign course and improve spontaneously within weeks of presentation. Some cases of focal eosinophilic myositis treated surgically have been reported [14]. Relapses are common and may occur several years after the initial episode [713]. Our patient was treated with high dose glucocorticoid and azathioprine because he had both the intestinal ulcerative lesion and focal eosinophilic myositis. After two weeks' treatment, muscle tenderness was resolved and size of mass decreased distinctively, which was also demonstrated by MRI of right upper arm.
We report a case of focal eosinophilic myositis in a patient with intestinal BD, which showed a good response to glucocorticoid therapy. Because there have been no published reports about BD accompanied by idiopathic eosinophilic myositis, it is not known whether these two diseases are correlated or not. Further studies are needed to elucidate the relation between BD and eosinophilic myopathy.
Figures and Tables
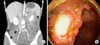 | Figure 1(A) Abdominal contrast-enhanced computed tomography shows a wall thickening and mucosal enhancement at the terminal ileum (arrows), suggesting terminal ileitis. (B) Colonoscopy reveals a large well-demarcated ulcerative lesion with central yellowish exudate at the terminal ileum. |
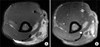 | Figure 2Magnetic resonance imaging of right upper arm. (A) An axial T2-weighted image demonstrates diffuse and irregular hyperintensity signal around a focal mass lesion in triceps muscle. (B) An axial gadolinium-enhanced T1-weighted image shows a focal mass lesion consisting of a well-defined rim of contrast enhancement (arrows) and a hypointense central area suspected of necrosis (arrowhead). |
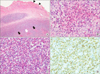 | Figure 3Histopathologic analysis of muscle. (A) Normal muscle structure (arrowheads) is observed sparsely, and the remainder is replaced by inflammatory cells and necrotic tissues (arrows) (H&E stain, ×40). (B) At higher magnification of the necrotic tissue (A, white arrow), the necrosis is surrounded by abundant inflammatory cells and eosinophils (H&E stain, ×400). (C, D) Infiltrations of eosinophils (C) and CD3+ T lymphocytes (D) are prominent in tissue (C: H&E stain, ×400; D: CD3 immunohistochemical stain, ×400). |
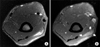 | Figure 4Follow-up magnetic resonance imaging (MRI) of right upper arm. (A) On axial T2-weighted image, the signal intensity and extent of the involved muscle have been distinctly decreased compared to the initial MRI findings. (B) An axial gadolinium-enhanced T1-weighted image reveals reduced size of focal mass lesion (arrows) and disappearance of internal necrosis, which was evident in previous MRI. |
References
1. Sakane T, Takeno M, Suzuki N, Inaba G. Behçet's disease. N Engl J Med. 1999; 341:1284–1291.
2. Skef W, Hamilton MJ, Arayssi T. Gastrointestinal Behçet's disease: a review. World J Gastroenterol. 2015; 21:3801–3812.
3. Zou J, Shen Y, Ji DN, Zheng SB, Guan JL. Endoscopic findings of gastrointestinal involvement in Chinese patients with Behcet's disease. World J Gastroenterol. 2014; 20:17171–17178.
4. Bicer A. Musculoskeletal findings in Behcet's disease. Patholog Res Int. 2012; 2012:653806.
5. Akansel G, Akgoz Y, Ciftci E, Arslan A, Demirci A. MRI findings of myositis in Behçet disease. Skeletal Radiol. 2004; 33:426–428.
6. Sarui H, Maruyama T, Ito I, Yamakita N, Takeda N, Nose M, et al. Necrotising myositis in Behçet's disease: characteristic features on magnetic resonance imaging and a review of the literature. Ann Rheum Dis. 2002; 61:751–752.
7. Pickering MC, Walport MJ. Eosinophilic myopathic syndromes. Curr Opin Rheumatol. 1998; 10:504–510.
8. Selva-O'Callaghan A, Trallero-Araguás E, Grau JM. Eosinophilic myositis: an updated review. Autoimmun Rev. 2014; 13:375–378.
9. Trüeb RM, Pericin M, Winzeler B, Wüthrich B, Burg G. Eosinophilic myositis/perimyositis: frequency and spectrum of cutaneous manifestations. J Am Acad Dermatol. 1997; 37:385–391.
10. Kaufman LD, Kephart GM, Seidman RJ, Buhner D, Qvarfordt I, Nässberger L, et al. The spectrum of eosinophilic myositis. Clinical and immunopathogenic studies of three patients, and review of the literature. Arthritis Rheum. 1993; 36:1014–1024.
11. Hatemi G, Seyahi E, Fresko I, Talarico R, Hamuryudan V. One year in review 2016: Behçet's syndrome. Clin Exp Rheumatol. 2016; 34:6 Suppl 102. 10–22.
12. Hall FC, Krausz T, Walport MJ. Idiopathic eosinophilic myositis. QJM. 1995; 88:581–586.
13. Sladek GD, Vasey FB, Sieger B, Behnke DA, Germain BF, Espinoza LR. Relapsing eosinophilic myositis. J Rheumatol. 1983; 10:467–470.
14. Agrawal BL, Giesen PC. Eosinophilic myositis. An unusual cause of pseudotumor and eosinophilia. JAMA. 1981; 246:70–71.
15. Sharma P, Sharma MC. Resolving eosinophilic myositis: MR features. J Comput Assist Tomogr. 2001; 25:151–153.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download