Abstract
Objective
Interleukin (IL)-17 is a proinflammatory cytokine that has pleiotropic effects on multiple target cells and thereby contributes to the development of immunemediated inflammatory disorders. However, the role of IL-17 in the humoral immune response has not been clearly elucidated.
Methods
Mice deficient in IL-17A (IL-17A knockout [KO] mice) and wild type (WT) C57BL/6 mice were compared. Distinct B cell (mature/precursor and marginal zone/follicular) and plasma cell populations were compared using fluorescence-activated cell sorting (FACS) and confocal immunostaining. Immunoglobulin production was assessed by enzyme-linked immunosorbent assay.
Results
There was no difference in B cell and plasma cell populations between IL-17A KO and WT mice. However, after T cell-dependent antigen challenge, IL-17A KO mice produced lower levels of immunoglobulin (Ig)G1 than wild-type animals. IL-17A KO mice also showed reduced germinal center (GC) formation and lower expression of activation-induced cytidine deaminase, the essential enzyme for class switch recombination (CSR). IL-17 had no effect on the proliferation or survival of naïve B cells in in vitro functional studies. However, IL-17 treatment promoted naïve B cell differentiation into plasma cells in synergy with IL-4, although IL-17 alone had no effect.
REFERENCES
3. Raychaudhuri SP. Role of IL-17 in psoriasis and psoriatic arthritis. Clin Rev Allergy Immunol. 2013; 44:183–93.

4. Matusevicius D, Kivisäkk P, He B, Kostulas N, Ozenci V, Fredrikson S, et al. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler. 1999; 5:101–4.

5. Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002; 8:500–8.

6. Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-defi-cient mice. J Immunol. 2003; 171:6173–7.

7. Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006; 177:566–73.

8. Patel DD, Lee DM, Kolbinger F, Antoni C. Effect of IL-17A blockade with secukinumab in autoimmune diseases. Ann Rheum Dis. 2013; 72(Suppl 2):ii116–23.

9. Lopez-Olivo MA, Amezaga Urruela M, McGahan L, Pollono EN, Suarez-Almazor ME. Rituximab for rheumatoid arthritis. Cochrane Database Syst Rev. 2015; 1:CD007356.

10. Guillevin L, Pagnoux C, Karras A, Khouatra C, Aumaître O, Cohen P, et al. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med. 2014; 371:1771–80.

11. Xie S, Li J, Wang JH, Wu Q, Yang P, Hsu HC, et al. IL-17 activates the canonical NF-kappaB signaling pathway in autoimmune B cells of BXD2 mice to upregulate the expression of regulators of G-protein signaling 16. J Immunol. 2010; 184:2289–96.
12. Ding Y, Li J, Wu Q, Yang P, Luo B, Xie S, et al. IL-17RA is essential for optimal localization of follicular Th cells in the germinal center light zone to promote autoantibody-pro-ducing B cells. J Immunol. 2013; 191:1614–24.

13. Mitsdoerffer M, Lee Y, Jäger A, Kim HJ, Korn T, Kolls JK, et al. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc Natl Acad Sci U S A. 2010; 107:14292–7.

14. Corneth OB, Mus AM, Asmawidjaja PS, Klein Wolterink RG, van Nimwegen M, Brem MD, et al. Absence of interleukin-17 receptor a signaling prevents autoimmune inflammation of the joint and leads to a Th2-like phenotype in collagen-induced arthritis. Arthritis Rheumatol. 2014; 66:340–9.

15. Patakas A, Benson RA, Withers DR, Conigliaro P, McInnes IB, Brewer JM, et al. Th17 effector cells support B cell responses outside of germinal centres. PLoS One. 2012; 7:e49715.

16. Hsu HC, Yang P, Wang J, Wu Q, Myers R, Chen J, et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol. 2008; 9:166–75.
17. Snapper CM, Paul WE. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987; 236:944–7.
Figure 1.
Comparison of the B cell populations of wild type (WT) and interleukin (IL)-17 knockout (KO) mice. Various B cell subpopulations were compared using FACS analysis. (A) Amongst the B220+ B cells, the subpopulation of precursor B cells (IgD− IgM−), mature B cells (IgD+ IgM−) (left), plasma B cells (CD138+) (middle), follicular B (FOB) cells (CD21 low CD23+), and marginal zone B (MZB) cells (CD21+ CD23 low) (right) were compared. The top panel shows WT and the bottom panel shows IL-17 KO. (B) The number of cells measured by FACS. Data are presented as mean±standard error of the mean. Ig: immunoglobulin, SSC: side scatter.
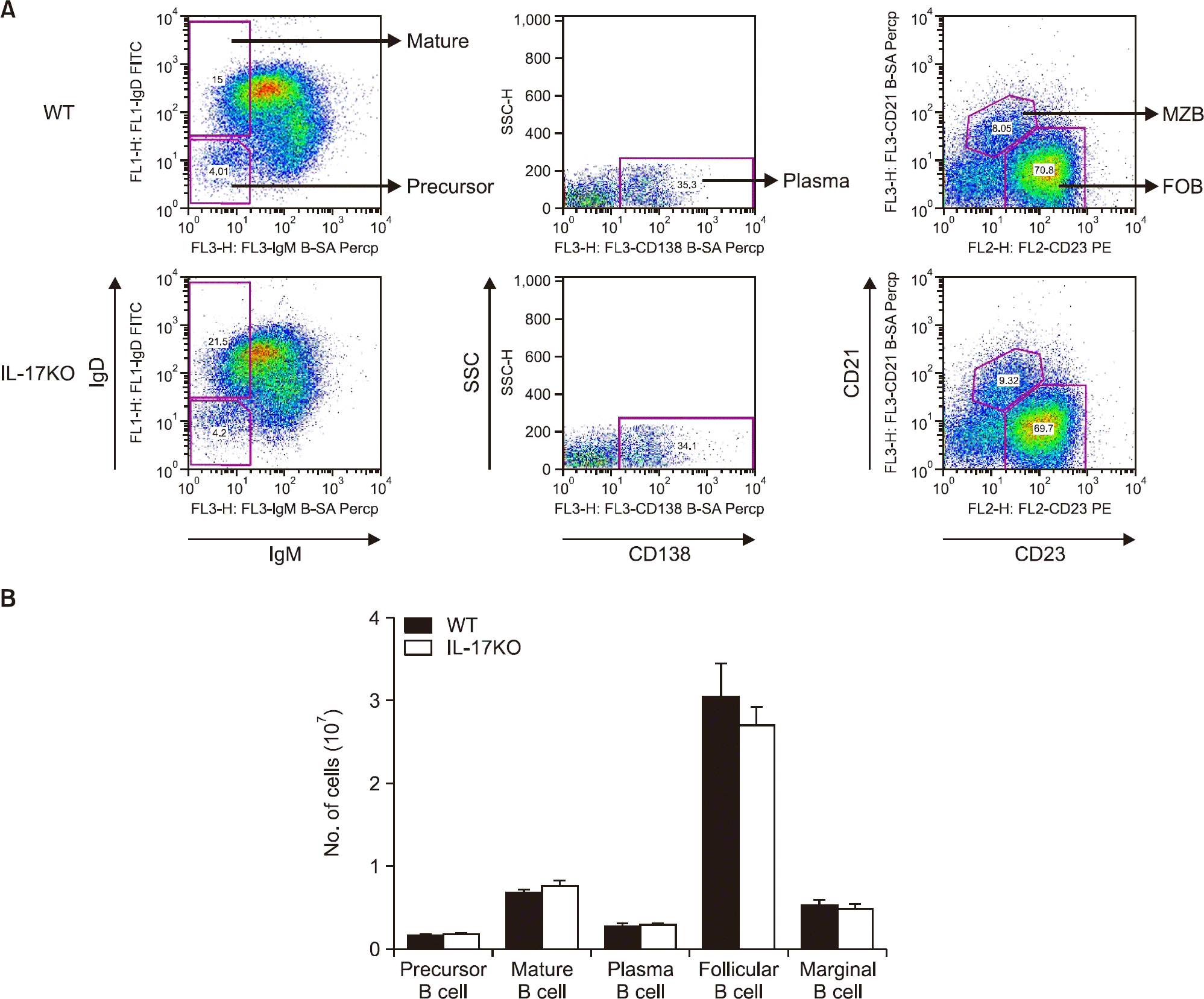
Figure 2.
Interleukin (IL)-17 contributes to immunoglobulin (Ig) G1 production in mice. Plasma samples (A) and spleen cells (B) were obtained on day 14-post immunization. (A) The levels of anti-4-hydroxy-3-nitrophenacetyl (NP) immunoglobulin in the plasma were measured by enzyme-linked immunosorbent assay (immunostaining, ×400). (B) The levels of total IgG1 positive splenic B cells were measured by FACS analysis. Data are presented as mean±standard error of the mean. WT: wild type, KO: knockout, AID: activation-induced cytidine deaminase. *p-value<0.05.
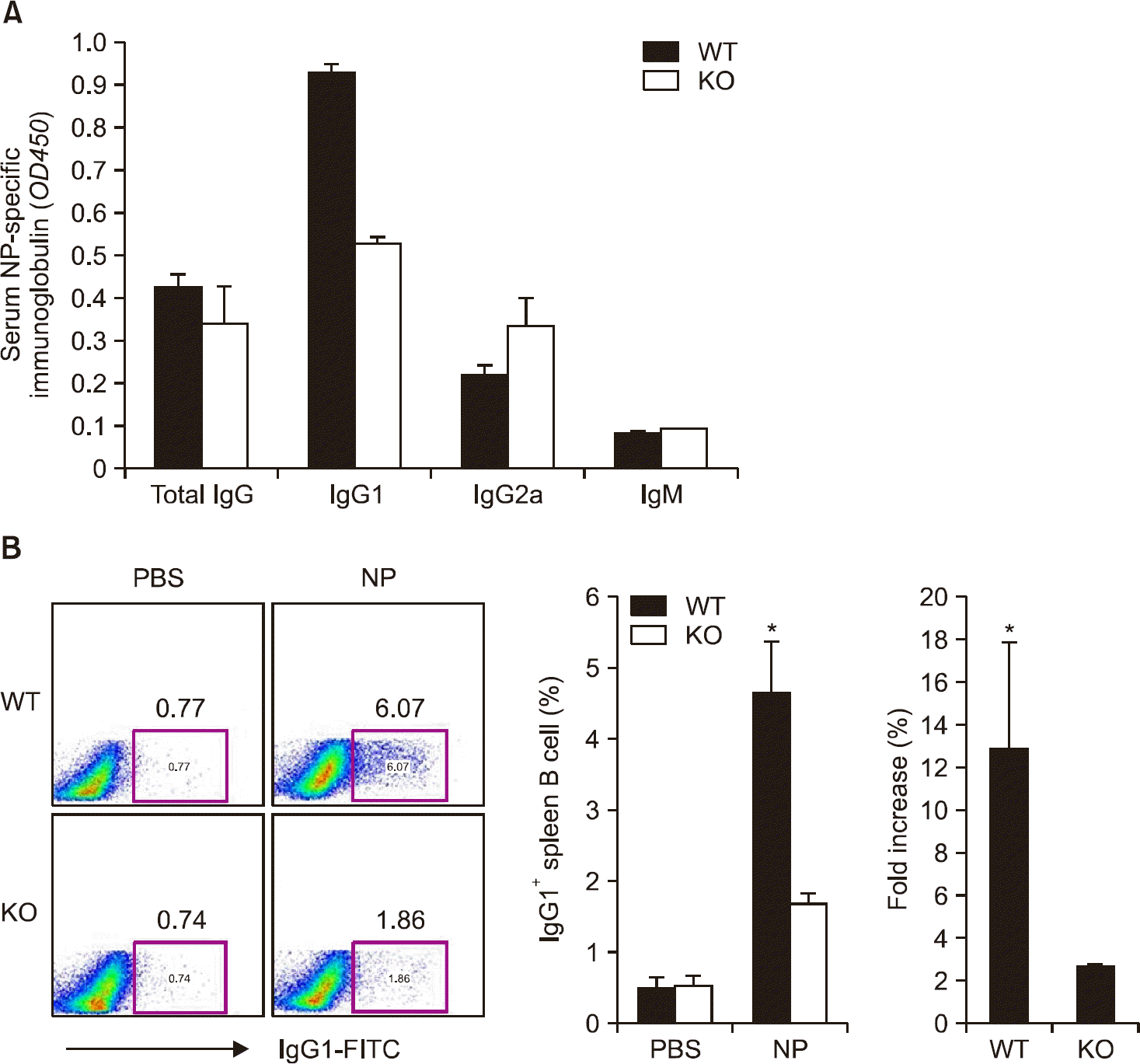
Figure 3.
Reduced germinal center formation in interleukin (IL)-17 knockout (KO) mice. Spleen tissue was obtained 14 days post immunization. (A) The number of germinal centers was observed on a cross section field using confocal microscopy (immunostaining, ×400). (B) Immunostaining for immunoglobulin (Ig)G1, CD4, and GL-7 was performed. Data are presented as mean±standard error of the mean. WT: wild type, NP: 4-hydroxy-3-nitrophenacetyl. *p-value<0.05.
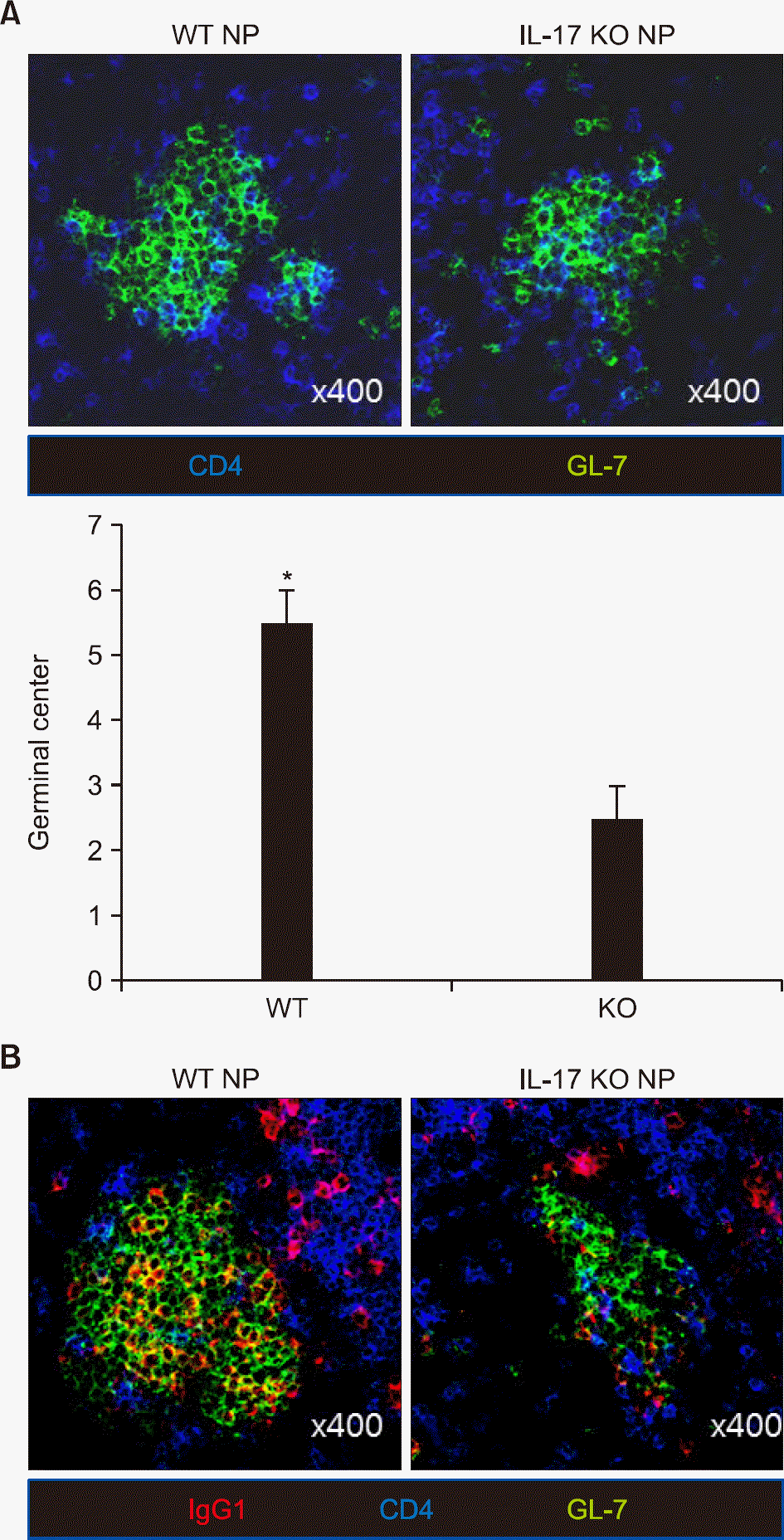
Figure 4.
The expression of activation-induced cytidine deaminase (AID) is decreased in the splenocytes of interleukin (IL)-17 knockout (KO) mice. The spleens (A) and splenocytes (B) were obtained on day 14-post immunization. (A) Immunostaining for AID, CD4, and GL-7 was performed. (B) The expression of AID in splenocytes was determined by reverse transcription-polymerase chain reaction. Data are presented as mean±standard error of the mean. Representative data are presented. WT: wild type, NP: 4-hydroxy-3-nitrophenacetyl.
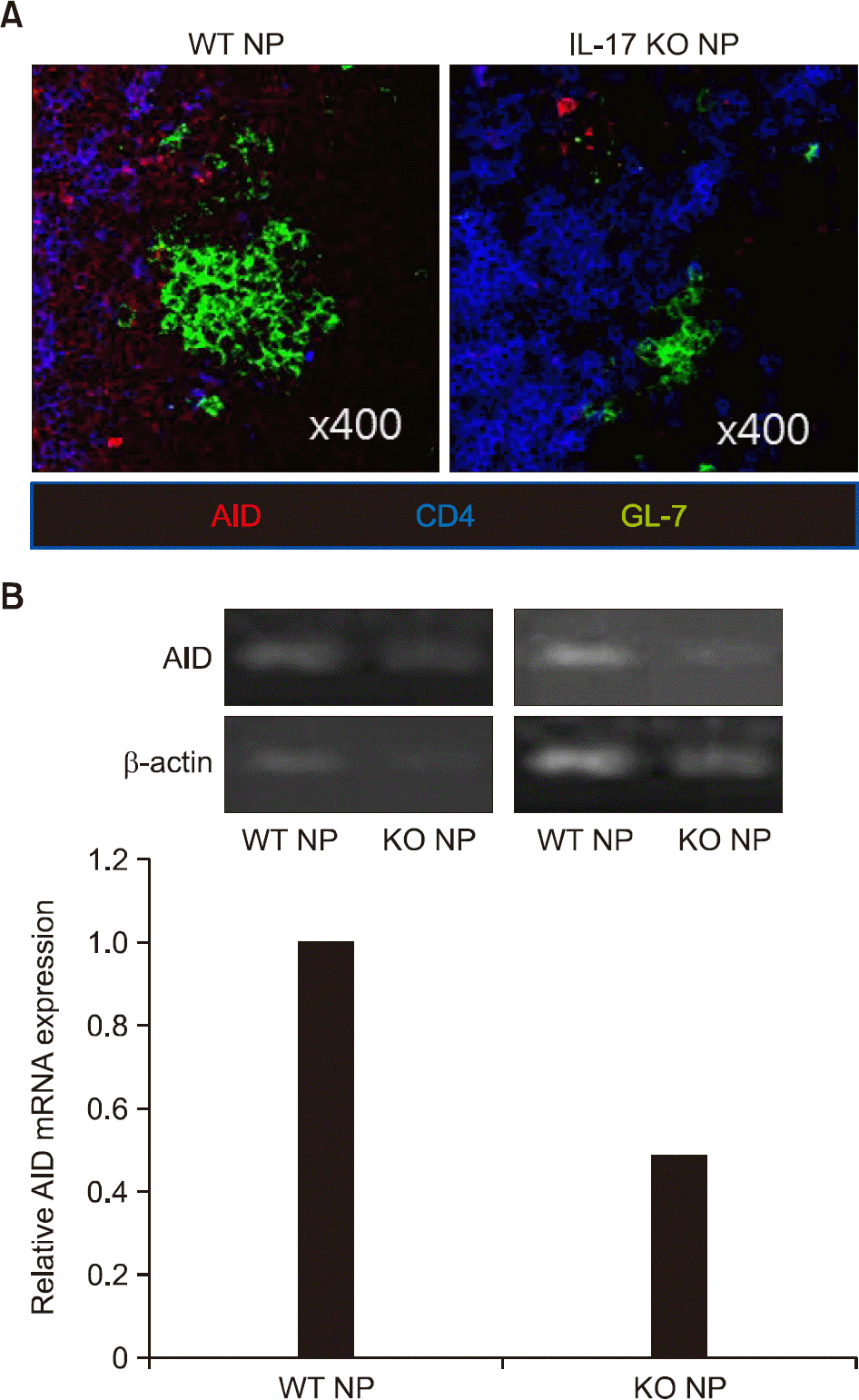
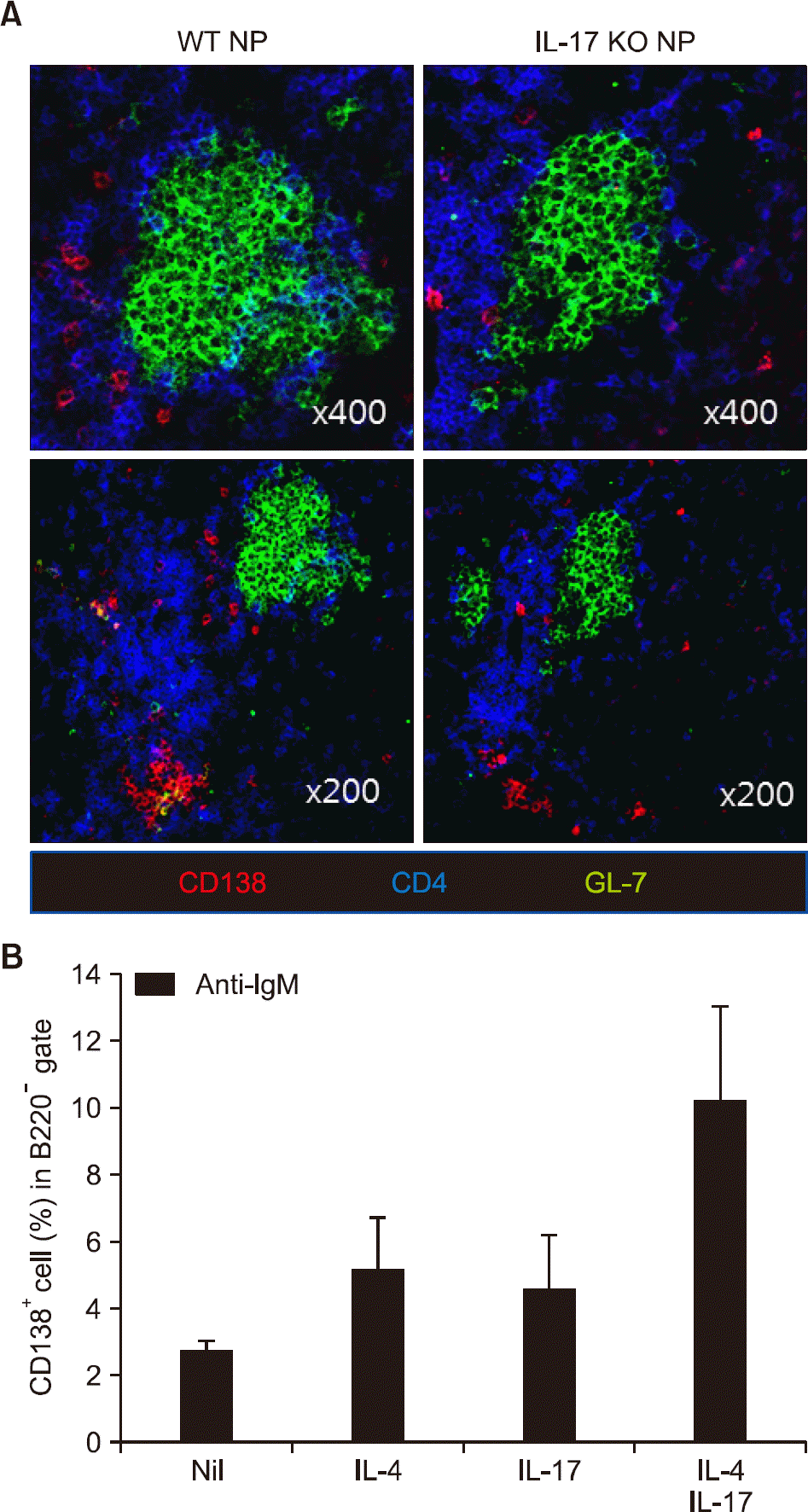
Figure 5.
Interleukin (IL)-17 enhances plasma cell differentiation in synergy with IL-4. (A) Spleens were obtained on day 14 after immunization. Immunostaining for CD138, CD4, and GL-7 was performed. (B) Resting B cells were isolated from untreated wild type (WT) mice and cultured with anti-immunoglobulin (Ig)M (5 μ g/mL) in the presence or absence of IL-4 (5 ng/mL) and/or IL-17 (10 ng/mL) for 5 days. Cells were harvested and the frequency of CD138+ B220− cells was measured using FACS. Data are presented as mean±standard error of the mean. NP: 4-hydroxy-3-nitrophenacetyl, KO: knockout.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download