Abstract
Objective
Failure of first-line anti-tumor necrosis factor (TNF) agents in in rheumatoid arthritis patients leads to decisions among second-line biologic agents. To better inform these decisions, the therapeutic effectiveness of rituximab is compared with other second-line biologic agents in this observational study.
Methods
Between November 2011 and December 2014, study subjects were observed for 12 month periods. Patients with an inadequate response to initial anti-TNF agent received either rituximab or alternative anti-TNF agents (adalimumab/etanercept/infliximab) based on the preference of patients and physicians. The efficacy end point of this study was the change in 28-joint count Disease Activity Score (DAS28) at six and 12 months from baseline. Safety data were also collected.
Results
Ninety patients were enrolled in the study. DAS28 at six months did not change significantly whether the patients were treated with rituximab or alternative anti-TNF agents in intention-to-treat analysis (n=34, −1.63±0.30 vs. n=31, −2.05±0.34) and standard population set analysis (n=31, −1.51±0.29 vs. n=24, −2.21±0.34). Similarly, the change in DAS28 at 12 months did not reach statistical significance (−1.82±0.35 in the rituximab vs. −2.34±0.44 in the alternative anti-TNF agents, p=0.2390). Furthermore, the incidences of adverse events were similar between two groups (23.5% for rituximab group vs. 25.8% for alternative anti-TNF agents group, p=0.7851).
Go to : 
REFERENCES
1. Maciejewska Rodrigues H, Jüngel A, Gay RE, Gay S. Innate immunity, epigenetics and autoimmunity in rheumatoid arthritis. Mol Immunol. 2009; 47:12–8.
2. Korea Ministry of Health and Welfare and Korea Centers for Disease Control & Prevention. Korea Health Statistics. 2009; Korea National Health and Nutrition Examination Survey (KNHANESIV-3). 2009, p.557.
4. Kim YJ, Choi CB, Sung YK, Lee H, Bae SC. Characteristics of Korean patients with RA: a single center cohort study. J Korean Rheum Assoc. 2009; 16:204–12.

5. 2009 National Health Insurance Statistical Yearbook. Seoul, Korea National Health Insurance Corporation. Health Insurance Review & Assessment Service;2010. p. 472.
6. American College of Rheumatology Subcommittee on Rheumatoid Arthritis Guidelines. Guidelines for the management of rheumatoid arthritis: 2002 Update. Arthritis Rheum. 2002; 46:328–46.
7. Kahlenberg JM, Fox DA. Advances in the medical treatment of rheumatoid arthritis. Hand Clin. 2011; 27:11–20.

8. Redlich K, Schett G, Steiner G, Hayer S, Wagner EF, Smolen JS. Rheumatoid arthritis therapy after tumor necrosis factor and interleukin-1 blockade. Arthritis Rheum. 2003; 48:3308–19.

9. Lipsky PE, van der Heijde DM, St Clair EW, Furst DE, Breedveld FC, Kalden JR, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000; 343:1594–602.
10. Weinblatt ME, Kremer JM, Bankhurst AD, Bulpitt KJ, Fleischmann RM, Fox RI, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor: Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999; 340:253–9.
11. Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003; 48:35–45.
12. Genovese MC, Schiff M, Luggen M, Becker JC, Aranda R, Teng J, et al. Efficacy and safety of the selective costimulation modulator abatacept following 2 years of treatment in patients with rheumatoid arthritis and an inadequate response to anti-tumour necrosis factor therapy. Ann Rheum Dis. 2008; 67:547–54.

13. Finckh A, Simard JF, Gabay C, Guerne PA. Evidence for differential acquired drug resistance to anti-tumour necrosis factor agents in rheumatoid arthritis. Ann Rheum Dis. 2006; 65:746–52.

14. Smolen JS, Landewé R, Breedveld FC, Dougados M, Emery P, Gaujoux-Viala C, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis. 2010; 69:964–75.
15. Emery P, Gottenberg JE, Rubbert-Roth A, Sarzi-Puttini P, Choquette D, Taboada VM, et al. Rituximab versus an alternative TNF inhibitor in patients with rheumatoid arthritis who failed to respond to a single previous TNF inhibitor: SWITCH-RA, a global, observational, comparative effectiveness study. Ann Rheum Dis. 2015; 74:979–84.

16. Chandran B, Goel A. A randomized, pilot study to assess the efficacy and safety of curcumin in patients with active rheumatoid arthritis. Phytother Res. 2012; 26:1719–25.

17. NICE technology appraisal guidance 195. Adalimumab, etanercept, infliximab, rituximab and abatacept for the treatment of rheumatoid arthritis after the failure of a TNF inhibitor: Part review of NICE technology appraisal guidance 36. Review of NICE technology appraisal guidance 126 and 141. August 2010. London. National Institute for Health and Clinical Excellence;2010.
18. Finckh A, Ciurea A, Brulhart L, Kyburz D, Möller B, Dehler S, et al. B cell depletion may be more effective than switching to an alternative anti-tumor necrosis factor agent in rheumatoid arthritis patients with inadequate response to anti-tumor necrosis factor agents. Arthritis Rheum. 2007; 56:1417–23.

19. Finckh A, Ciurea A, Brulhart L, Möller B, Walker UA, Courvoisier D, et al. Which subgroup of patients with rheumatoid arthritis benefits from switching to rituximab versus alternative anti-tumour necrosis factor (TNF) agents after previous failure of an anti-TNF agent? Ann Rheum Dis. 2010; 69:387–93.

20. Sung YK, Cho SK, Choi CB, Bae SC. Prevalence and incidence of rheumatoid arthritis in South Korea. Rheumatol Int. 2013; 33:1525–32.

21. Burroughs VJ, Maxey RW, Levy RA. Racial and ethnic differences in response to medicines: towards individualized pharmaceutical treatment. J Natl Med Assoc. 2002; 94(10 Suppl):1–26.
22. Furst DE, Gaylis N, Bray V, Olech E, Yocum D, Ritter J, et al. Open-label, pilot protocol of patients with rheumatoid arthritis who switch to infliximab after an incomplete response to etanercept: the opposite study. Ann Rheum Dis. 2007; 66:893–9.

23. Gomez-Reino JJ, Carmona L. Switching TNF antagonists in patients with chronic arthritis: an observational study of 488 patients over a four-year period. Arthritis Res Ther. 2006; 8:R29.
24. Rubbert-Roth A, Finckh A. Treatment options in patients with rheumatoid arthritis failing initial TNF inhibitor therapy: a critical review. Arthritis Res Ther. 2009; 11(Suppl 1):S1.

25. van Vollenhoven RF, Emery P, Bingham CO 3rd, Keystone EC, Fleischmann RM, Furst DE, et al. Long-term safety of rituximab in rheumatoid arthritis: 9.5-year follow-up of the global clinical trial programme with a focus on adverse events of interest in RA patients. Ann Rheum Dis. 2013; 72:1496–502.

Go to : 
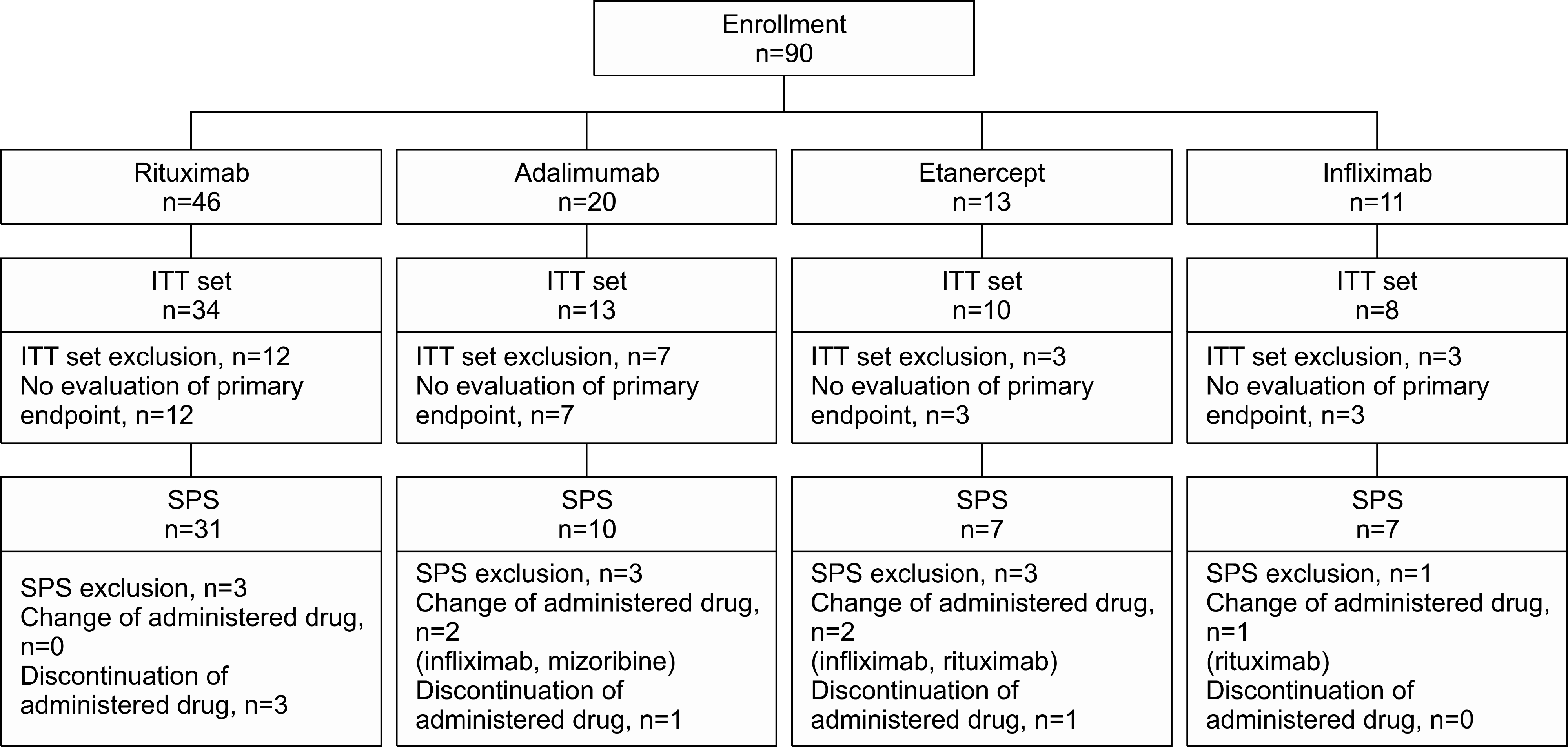 | Figure 1.Study participation diagram. ITT: intention-to-treat, SPS: standard population set. |
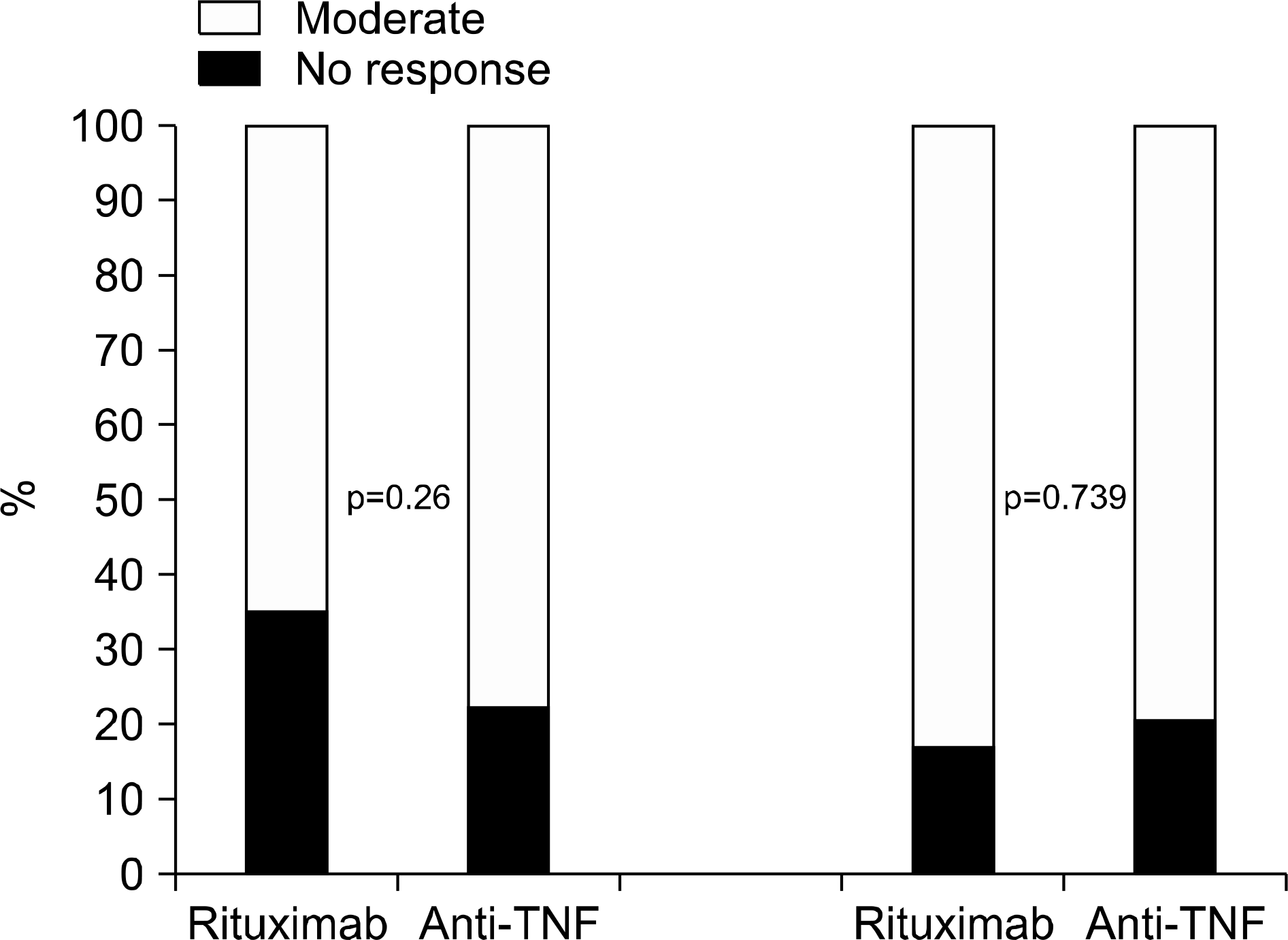 | Figure 2.European League Against Rheumatism response after 6 months and 12 months of treatment with rituximab and alternative anti-tumor necrosis factor (TNF) agents. |
Table 1.
Demographic and disease information (intention-to-treat set)
| Variable | Rituximab group (n=34) | Other anti-TNF agents group (n=31) | p-value | ||
|---|---|---|---|---|---|
| Age (yr) | Mean±SD | 55.62±11.92 | 52.74±14.36 | 0.3815§ | |
| Median | 60.00 | 55.00 | |||
| 20∼29 | 0 (0.0) | 2 (6.5) | 0.2226‡ | ||
| 30∼39 | 4 (11.8) | 4 (12.9) | |||
| 40∼49 | 7 (20.6) | 7 (22.6) | |||
| 50∼59 | 6 (17.7) | 7 (22.6) | |||
| 60∼69 | 15 (44.1) | 6 (19.4) | |||
| 70∼79 | 2 (5.9) | 5 (16.1) | |||
| Gender | Male | 9 (26.5) | 5 (16.1) | 0.3111† | |
| Female | 25 (73.5) | 26 (83.9) | |||
| Smoking status | Smoker | 5 (14.7) | 0 (0.0) | 0.1040‡ | |
| Non smoker | 26 (76.5) | 27 (87.1) | |||
| UK | 3 (8.8) | 4 (12.9) | |||
| RF status at baseline | Positive | 18 (52.9) | 19 (61.3) | 0.7599‡ | |
| Negative | 4 (11.8) | 2 (6.5) | |||
| ND or UK | 12 (35.3) | 10 (32.3) | |||
| Anti-CCP status at baseline | e Positive | 15 (44.1) | 15 (48.4) | 1.0000‡ | |
| Negative | 3 (8.8) | 2 (6.5) | |||
| ND or UK | 16 (47.1) | 14 (45.2) | |||
| Arthroplasty history∗ | Hip | Yes | 3 (8.8) | 1 (3.2) | 0.1496‡ |
| No | 30 (88.2) | 25 (80.7) | |||
| UK | 1 (2.9) | 5 (16.1) | |||
| Knee | Yes | 1 (2.9) | 4 (12.9) | 0.3619‡ | |
| No | 30 (88.2) | 25 (80.7) | |||
| UK | 3 (8.8) | 2 (6.5) | |||
| Elbow | Yes | 0 (0.0) | 2 (6.5) | 0.5467‡ | |
| No | 30 (88.2) | 25 (80.7) | |||
| UK | 4 (11.8) | 4 (12.9) | |||
| Shoulder | Yes | 0 (0.0) | 0 (0.0) | 0.4995‡ | |
| No | 30 (88.2) | 25 (80.7) | |||
| UK | 4 (11.8) | 6 (19.4) | |||
Table 2.
Prescription status at 12 months from second-line biological agents started for each treatment group (intention-to-treat set)
| Prescription status | Rituximab group (n=34) O | Other anti-TNF agents group (n=31) | p-value | |
|---|---|---|---|---|
| Initial anti-TNF agents | n | 34 | 31 | |
| Adalimumab | 17 (50.0) | 15 (48.4) | 1.0000† | |
| Etanercept | 14 (41.2) | 13 (41.9) | ||
| Infliximab | 3 (8.8) | 3 (9.7) | ||
| Reason for discontinuation of initial anti-TNF agents | n | 34 | 31 | |
| No response | 9 (26.5) | 7 (22.6) | 0.8529† | |
| Loss of response | 23 (67.7) | 21 (67.7) | ||
| Side effects | 2 (5.9) | 3 (9.7) | ||
| Status of second-line biologics at 6 months | n | 34 | 31 | |
| Maintain | 31 (91.2) | 24 (77.4) | 0.0620† | |
| Change | 0 (0.0) | 5 (16.1) | ||
| Discontinuation | 3 (8.8) | 2 (6.5) | ||
| Reason for discontinuation of second-line biologics | n | 3 | 7 | |
| No response | 3 (100.0) | 2 (28.6) | 0.2083† | |
| Loss of response | 0 (0.0) | 3 (42.9) | ||
| Side effects | 0 (0.0) | 2 (28.6) | ||
| Status of second-line biologics at 12 months | n∗ | 29 | 21 | |
| Maintain | 24 (82.8) | 17 (81.0) | 0.3389† | |
| Change | 4 (13.8) | 1 (4.8) | ||
| Discontinuation | 1 (3.5) | 3 (14.3) | ||
| Reason for discontinuation of second-line biologics | n | 5 | 4 | |
| No response | 1 (20.0) | 1 (25.0) | 1.0000† | |
| Loss of response | 3 (60.0) | 2 (50.0) | ||
| Side effects | 1 (20.0) | 0 (0.0) | ||
| Other | 0 (0.0) | 1 (25.0) | ||
Table 3.
Change in DAS28 at 6 months comparing to baseline for each treatment group
| DAS28 | Rituximab | Other TNF inhibitors | p-value | |
|---|---|---|---|---|
| ITT | n=34 | n=31 | ||
| Baseline | n | 34 | 31 | |
| Mean±SD | 6.35±1.14 | 5.54±1.14 | 0.0056∗ | |
| Median | 6.35 | 5.65 | ||
| Min, Max | 3.47, 8.35 | 3.37, 7.95 | ||
| 6 months | n | 34 | 31 | |
| Mean±SD | 4.46±1.63 | 3.74±1.40 | ||
| Median | 4.13 | 3.59 | ||
| Min, Max | 0.68, 7.54 | 1.21, 7.17 | ||
| Change | n | 34 | 31 | |
| Mean±SD | −1.89±1.73 | −1.80±1.53 | 0.3037† | |
| LS Mean±SE | −1.63±0.30 | −2.05±0.34 | ||
| Median | −1.91 | −1.83 | ||
| Min, Max | −6.09, 1.31 | −5.09, 1.13 | ||
| SPS | n=31 | n=24 | ||
| Baseline | n | 31 | 24 | |
| Mean±SD | 6.30±1.18 | 5.47±1.04 | 0.0089∗ | |
| Median | 6.10 | 5.49 | ||
| Min, Max | 3.47, 8.35 | 3.37, 7.95 | ||
| 6 months | n | 31 | 24 | |
| Mean±SD | 4.56±1.51 | 3.56±1.33 | ||
| Median | 4.13 | 3.37 | ||
| Min, Max | 1.59, 7.54 | 1.21, 6.98 | ||
| Change | n | 31 | 24 | |
| Mean±SD | −1.74±1.59 | −1.91±1.53 | 0.0951† | |
| LS Mean±SE | −1.51±0.29 | −2.21±0.34 | ||
| Median | −1.86 | −1.97 | ||
| Min, Max | −4.76, 1.31 | −5.09, 1.13 | ||
Table 4.
Change in DAS28 at 12 months comparing to baseline for each treatment group
| DAS28 | Rituximab group (n=24) | Other anti-TNF agents group (n=17) | p-value | |
|---|---|---|---|---|
| Baseline | n | 24 | 17 | |
| Mean±SD | 6.28±1.27 | 5.49±0.92 | 0.0341† | |
| Median | 6.13 | 5.65 | ||
| Min, Max | 3.47, 8.35 | 3.37, 6.98 | ||
| 12 months | n∗ | 22 | 16 | |
| Mean±SD | 3.85±1.37 | 3.10±1.07 | ||
| Median | 3.92 | 3.20 | ||
| Min, Max | 0.01, 6.99 | 1.21, 5.25 | ||
| Change | n∗ | 22 | 16 | |
| Mean±SD | −2.30±1.52 | −2.29±1.36 | 0.2390‡ | |
| LS Mean±SE | −1.82±0.35 | −2.34±0.44 | ||
| Median | −2.34 | −2.39 | ||
| Min, Max | −5.28, −0.04 | −4.70, 0.61 |
Change is difference between baseline and 12 months. DAS2: disease activity score in 28 joints, TNF: tumor necrosis factor, SD: standard deviation, LS: least squares, SE: standard error.
Table 5.
Adverse events on organ systems (intention-to-treat set)
| System Organ Class (preferred term) |
Rituximab group (n=34) |
Other anti-TNF agents group (n=31) |
||
|---|---|---|---|---|
| n* (%) | Event | n* (%) | Event | |
| Number of subjects with adverse event (0.7851†) | 8 (23.5) | 12 | 8 (25.8) | 15 |
| Gastrointestinal disorders | 1 (2.9) | 1 | 4 (12.9) | 5 |
| Diarrhea | 0 (0.0) | 0 | 2 (6.5) | 2 |
| Abdominal discomfort | 1 (2.9) | 1 | 0 (0.0) | 0 |
| Abdominal pain upper | 0 (0.0) | 0 | 1 (3.2) | 1 |
| Mouth ulceration | 0 (0.0) | 0 | 1 (3.2) | 1 |
| Nausea | 0 (0.0) | 0 | 1 (3.2) | 1 |
| Nausea Infections and infestations | 0 (0.0)2 (5.9) | 0 2 | 1 (3.2)3 (9.7) | 1 4 |
| Upper respiratory tract infection | 1 (2.9) | 1 | 1 (3.2) | 2 |
| Pneumonia | 1 (2.9) | 1 | 0 (0.0) | 0 |
| Tuberculosis | 0 (0.0) | 0 | 1 (3.2) | 1 |
| Urethritis | 0 (0.0) | 0 | 1 (3.2) | 1 |
| Urethritis Skin and subcutaneous tissue disorders | 0 (0.0) 2 (5.9) | 0 4 | 1 (3.2)2 (6.5) | 1 2 |
| Erythema | 1 (2.9) | 1 | 0 (0.0) | 0 |
| Pruritus | 1 (2.9) | 1 | 0 (0.0) | 0 |
| Rash | 0 (0.0) | 0 | 1 (3.2) | 1 |
| Skin lesion | 1 (2.9) | 1 | 0 (0.0) | 0 |
| Skin ulcer | 0 (0.0) | 0 | 1 (3.2) | 1 |
| Urticaria | 1 (2.9) | 1 | 0 (0.0) | 0 |
| General disorders and administration site conditions | 0 (0.0) | 0 | 2 (6.5) | 2 |
| Oedema | 0 (0.0) | 0 | 1 (3.2) | 1 |
| Pyrexia | 0 (0.0) | 0 | 1 (3.2) | 1 |
| Respiratory, thoracic and mediastinal disorders | 1 (2.9) | 1 | 1 (3.2) | 1 |
| Cough | 0 (0.0) | 0 | 1 (3.2) | 1 |
| Dyspnea | 1 (2.9) | 1 | 0 (0.0) | 0 |
| Eye disorders | 2 (5.9) | 2 | 0 (0.0) | 0 |
| Dry eye | 1 (2.9) | 1 | 0 (0.0) | 0 |
| Iritis | 1 (2.9) | 1 | 0 (0.0) | 0 |
| Immune system disorders | 1 (2.9) | 1 | 0 (0.0) | 0 |
| Anaphylactoid reaction | 1 (2.9) | 1 | 0 (0.0) | 0 |
| Musculoskeletal and connective tissue disorders | 0 (0.0) | 0 | 1 (3.2) | 1 |
| Back pain | 0 (0.0) | 0 | 1 (3.2) | 1 |
| Psychiatric disorders | 1 (2.9) | 1 | 0 (0.0) | 0 |
| Delirium | 1 (2.9) | 1 | 0 (0.0) | 0 |
| Number of subjects with adverse drug reaction (0.8205†) | 4 (11.8) | 5 | 5 (16.1) | 7 |
| Skin and subcutaneous tissue disorders | 2 (5.9) | 3 | 2 (6.5) | 2 |
| Erythema | 1 (2.9) | 1 | 0 (0.0) | 0 |
| Pruritus | 1 (2.9) | 1 | 0 (0.0) | 0 |
| Rash | 0 (0.0) | 0 | 1 (3.2) | 1 |
| Skin ulcer | 0 (0.0) | 0 | 1 (3.2) | 1 |
| Urticaria | 1 (2.9) | 1 | 0 (0.0) | 0 |
| Infections and infestations | 1 (2.9) | 1 | 2 (6.5) | 2 |
| Pneumonia | 1 (2.9) | 1 | 0 (0.0) | 0 |
| Tuberculosis | 0 (0.0) | 0 | 1 (3.2) | 1 |
| Tuberculosis Urethritis | 0 (0.0)0 (0.0) | 0 0 | 1 (3.2)1 (3.2) | 1 1 |
| Gastrointestinal disorders | 0 (0.0) | 0 | 2 (6.5) | 2 |
| Diarrhea | 0 (0.0) | 0 | 1 (3.2) | 1 |
| Nausea | 0 (0.0) | 0 | 1 (3.2) | 1 |
| Immune system disorders | 1 (2.9) | 1 | 0 (0.0) | 0 |
| Anaphylactoid reaction | 1 (2.9) | 1 | 0 (0.0) | 0 |
| Respiratory, thoracic and mediastinal disorders | 0 (0.0) | 0 | 1 (3.2) | 1 |
| Cough | 0 (0.0) | 0 | 1 (3.2) | 1 |
| Number of subjects with serious adverse reaction (0.8330†) | 3 (8.8) | 3 | 0 (0.0) | 0 |
| Immune system disorders | 1 (2.9) | 1 | 0 (0.0) | 0 |
| Anaphylactoid reaction | 1 (2.9) | 1 | 0 (0.0) | 0 |
| Anaphylactoid reaction Infections and infestations | 1 (2.9)1 (2.9) | 1 1 | 0 (0.0) 0 (0.0) | 0 0 |
| Pneumonia | 1 (2.9) | 1 | 0 (0.0) | 0 |
| Respiratory, thoracic and mediastinal disorders | 1 (2.9) | 1 | 0 (0.0) | 0 |
| Dyspnea | 1 (2.9) | 1 | 0 (0.0) | 0 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download