Abstract
Objective
This study assessed the efficacy and safety of secukinumab and adalimumab in patients with active ankylosing spondylitis (AS).
Methods
A Bayesian network meta-analysis was performed with direct and indirect data collected from randomized controlled trials (RCTs) of efficacy and safety of secukinumab 75 mg, 150 mg and adalimumab 40 mg in patients with active AS.
Results
Five RCTs (1,483 patients) met the inclusion criteria. The Assessment in Spondyloarthritis International Society response criteria of ≥20% (ASAS20) response rate was significantly higher in the adalimumab 40 mg (Odds ratio [OR], 4.26; 95% credible interval [CrI], 2.09∼8.08), secukinumab 150 mg (OR, 3.35; 95% CrI, 1.73∼6.56), and 75 mg dose (OR, 2.44; 95% CrI, 1.06∼5.05) than with placebo. The ranking probability based on the surface under the cumulative ranking curve (SUCRA) indicated that adalimumab 40 mg had the highest probability of being the best treatment for achieving an ASAS20 response (SUCRA=0.8753), followed by secukinumab 150 mg (SUCRA=0.7051), secukinumab 75 mg (SUCRA=0.4113), and placebo (SUCRA=0.0083). The ASAS40 response rate distribution pattern was similar to the ASAS20 response rate. However, the number of serious adverse events did not differ significantly among the treatment options.
Go to : 
REFERENCES
1. Brown MA, Wordsworth BP, Reveille JD. Genetics of ankylosing spondylitis. Clin Exp Rheumatol. 2002; 20(6 Suppl 28):S43–9.
2. Park R, Kim TH, Ji JD. Gene expression profile in patients with axial spondyloarthritis: meta-analysis of publicly accessible microarray datasets. J Rheum Dis. 2016; 23:363–72.

3. Braun J, van den Berg R, Baraliakos X, Boehm H, BurgosVargas R, Collantes-Estevez E, et al. 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis. 2011; 70:896–904.

4. Braun J, Bollow M, Neure L, Seipelt E, Seyrekbasan F, Herbst H, et al. Use of immunohistologic and in situ hybrid-ization techniques in the examination of sacroiliac joint biopsy specimens from patients with ankylosing spondylitis. Arthritis Rheum. 1995; 38:499–505.

5. Baeten D, Baraliakos X, Braun J, Sieper J, Emery P, van der Heijde D, et al. Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2013; 382:1705–13.

6. Shen H, Goodall JC, Hill Gaston JS. Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum. 2009; 60:1647.

7. Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med. 2014; 371:326–38.
8. Kivitz A, Blanco R, Maradiaga M, Sieper J, Tahir H, Andersson M, et al. Secukinumab provides sustained improvement in physical, function, health-related quality of life, fatigue, and work productivity in patients with active psoriatic arthritis: 52-week results from an international phase 3 trial. J Clin Rheumatol. 2016; 22:142–3.
9. Baeten D, Sieper J, Braun J, Baraliakos X, Dougados M, Emery P, et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med. 2015; 373:2534–48.

10. Huang F, Gu J, Zhu P, Bao C, Xu J, Xu H, et al. Efficacy and safety of adalimumab in Chinese adults with active ankylosing spondylitis: results of a randomised, controlled trial. Ann Rheum Dis. 2014; 73:587–94.

11. Lambert RG, Salonen D, Rahman P, Inman RD, Wong RL, Einstein SG, et al. Adalimumab significantly reduces both spinal and sacroiliac joint inflammation in patients with ankylosing spondylitis: a multicenter, randomized, doubleblind, placebo-controlled study. Arthritis Rheum. 2007; 56:4005–14.

12. van der Heijde D, Kivitz A, Schiff MH, Sieper J, Dijkmans BA, Braun J, et al. Efficacy and safety of adalimumab in patients with ankylosing spondylitis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2006; 54:2136–46.

13. Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ. 2005; 331:897–900.

14. Betts KA, Griffith J, Song Y, Mittal M, Joshi A, Wu EQ, et al. Network meta-analysis and cost per responder of tumor necrosis factor-α and interleukin inhibitors in the treatment of active ankylosing spondylitis. Rheumatol Ther. 2016; 3:323–36.

15. Chen C, Zhang X, Xiao L, Zhang X, Ma X. Comparative effectiveness of biologic therapy regimens for ankylosing spondylitis: a systematic review and a network meta-analysis. Medicine (Baltimore). 2016; 95:e3060.
16. Anderson JJ, Baron G, van der Heijde D, Felson DT, Dougados M. Ankylosing spondylitis assessment group preliminary definition of short-term improvement in ankylosing spondylitis. Arthritis Rheum. 2001; 44:1876–86.

17. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009; 151:264–9.

18. Brown S, Hutton B, Clifford T, Coyle D, Grima D, Wells G, et al. A microsoft-excel-based tool for running and critically appraising network meta-analyses–an overview and application of NetMetaXL. Syst Rev. 2014; 3:110.

19. Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multi-ple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011; 64:163–71.

20. Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med Decis Making. 2013; 33:641–56.
21. Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods. 2012; 3:98–110.

22. van Valkenhoef G, Lu G, de Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta-analysis. Res Synth Methods. 2012; 3:285–99.

23. Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012; 83:1583–90.

24. Kiltz U, Heldmann F, Baraliakos X, Braun J. Treatment of ankylosing spondylitis in patients refractory to TNF-inhibition: are there alternatives? Curr Opin Rheumatol. 2012; 24:252–60.
25. Leonardi CL, Kimball AB, Papp KA, Yeilding N, Guzzo C, Wang Y, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, doubleblind, placebo-controlled trial (PHOENIX 1). Lancet. 2008; 371:1665–74.

26. Ritchlin C, Haas-Smith SA, Hicks D, Cappuccio J, Osterland CK, Looney RJ. Patterns of cytokine production in psoriatic synovium. J Rheumatol. 1998; 25:1544–52.
Go to : 
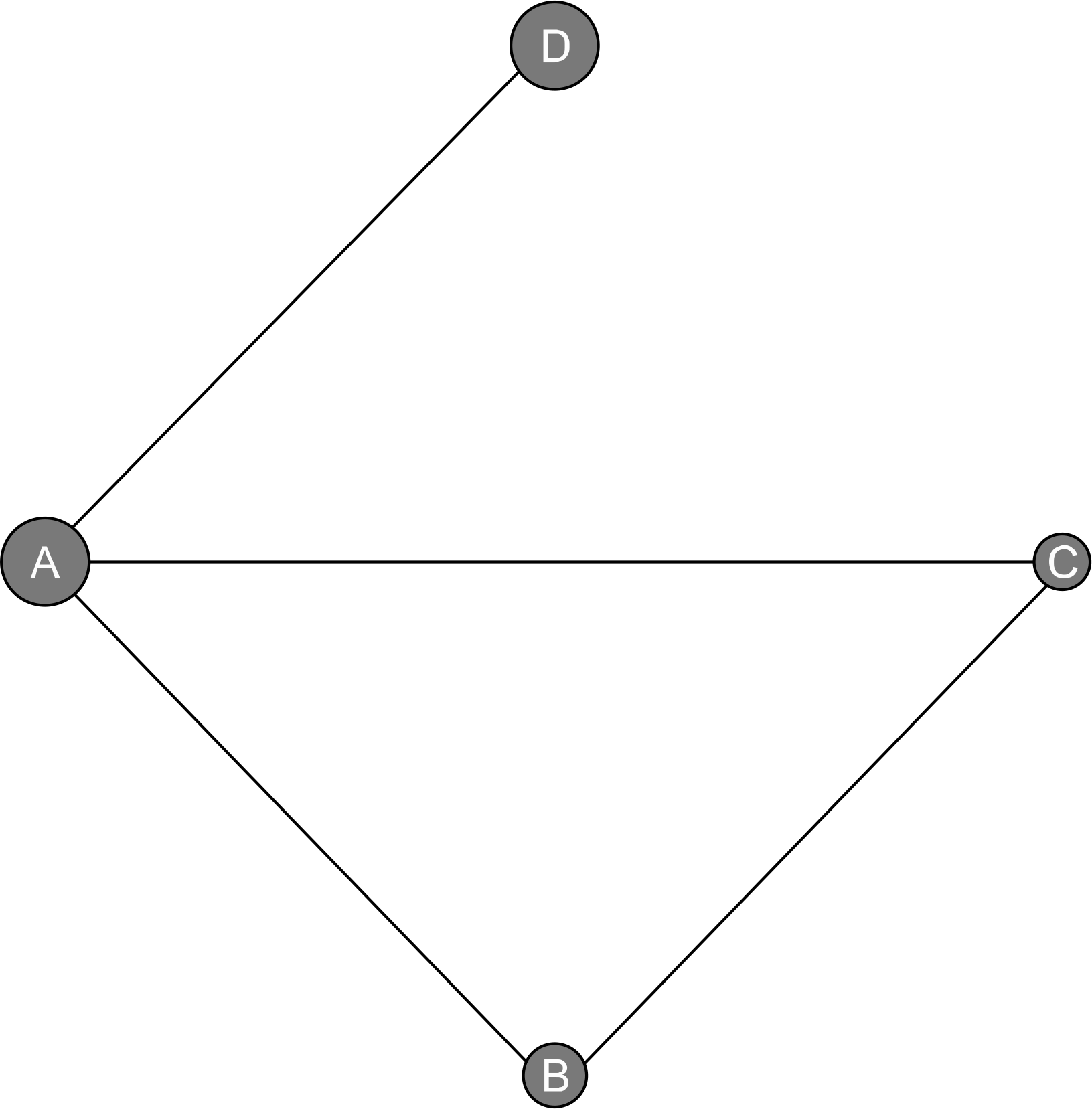 | Figure 2.Evidence network diagram of comparisons for network meta-analysis. The width of each edge is proportional to the number of randomized controlled trials comparing each pair of treatments, and the size of each treatment node is proportional to the number of randomized participants (sample size). (A) Placebo. (B) Secukinumab 150 mg. (C) Secukinumab 75 mg. (D) Adalimumab 40 mg. |
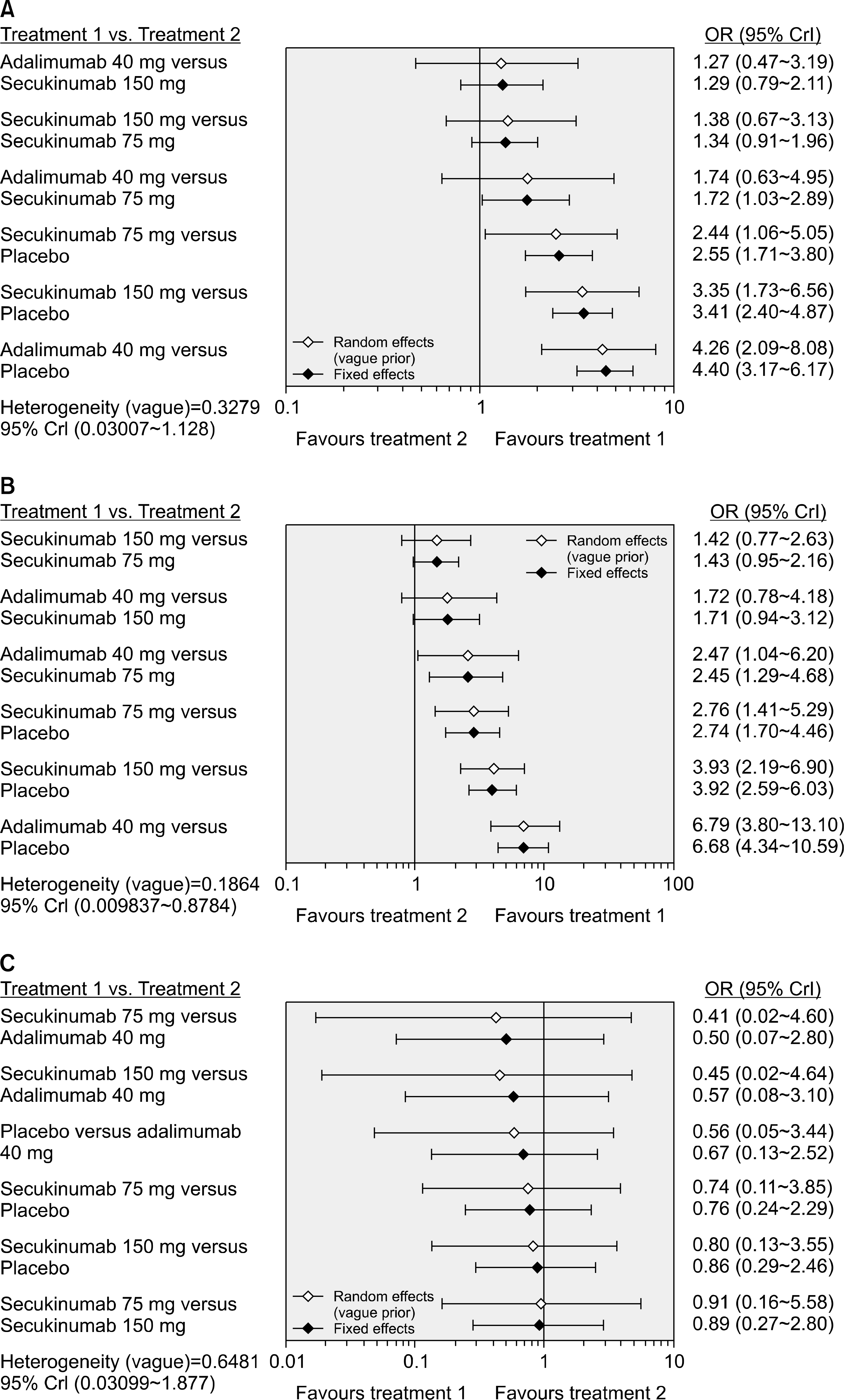 | Figure 3.Results of the Bayesian network meta-analysis of randomized controlled studies on the relative efficacy (A: ASAS20, B: ASAS40) and safety (C) of secukinumab and adalimumab. ASAS: Assessment in Spondyloarthritis International Society, OR: odds ratio, CrI: credible interval. |
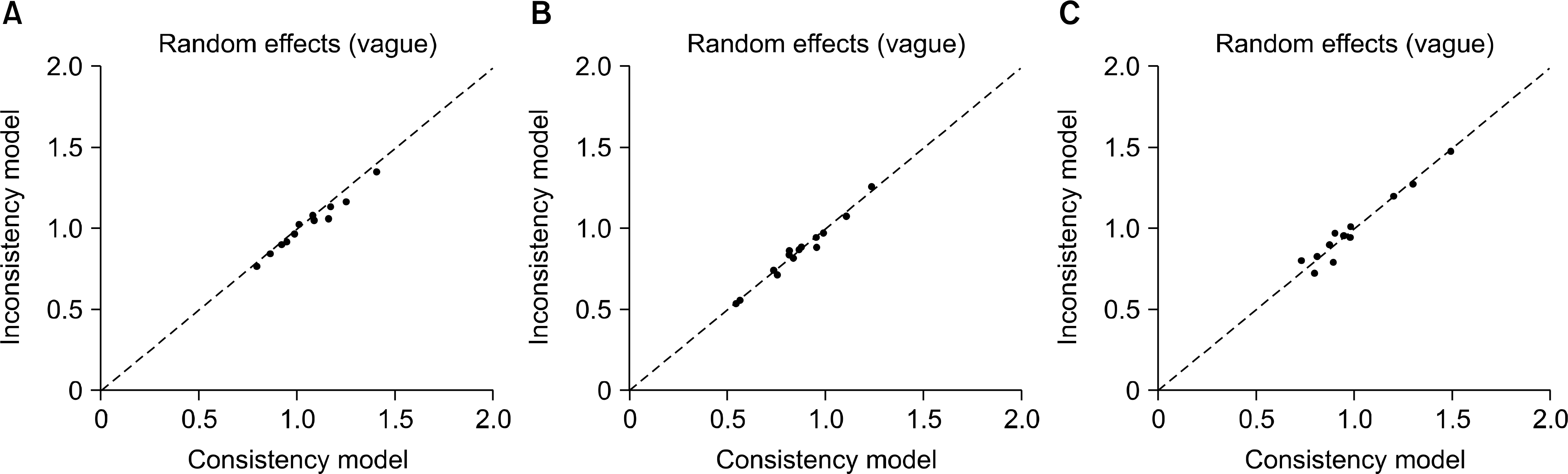 | Figure 4.Inconsistency plot for the efficacy (A: ASAS20, B: ASAS40) and safety (C) of secukinumab and adalimumab. Plot of the individual data points' posterior mean deviance contributions for the consistency model (horizontal axis) and the unrelated mean effects model (vertical axis) along with the line of equality. ASAS: Assessment in Spondyloarthritis International Society. |
Table 1.
Characteristics of individual studies included in the network meta-analysis
Values are presented as number. ASAS20: Assessment of Spondyloarthritis International Society 20 response criteria (improvement of ≥20% and absolute improvement of ≥1 unit [on a 10-unit scale] in at least three of the four main ASAS domains, with no worsening by ≥20% in the remaining domain) [16], NA: not available.
Table 2.
Characteristics of individual studies included in the network meta-analysis
| Study | Drugs | Age (yr) | Disease duration (yr) | Male | HLA-B27 | BASDAI |
|---|---|---|---|---|---|---|
| Kivitz, 2016 [8] | Secukinumab 150 mg | 42.1∼42.9∗ | 5.2∼6.0∗ | NA | NA | NA |
| Placebo | 42.1∼42.9∗ | 5.2∼6.0∗ | NA | NA | NA | |
| Baeten-1, 2015 [9] | Secukinumab 150 mg | 40.1±11.6 | 6.5±6.9 | 67 | 69 | 6.4±1.6 |
| Secukinumab 75 mg | 42.3±13.2 | 7.9±9.7 | 71 | 80 | 6.1±1.4 | |
| Placebo | 43.1±12.4 | 8.3±8.9 | 70 | 74 | 6.5±1.5 | |
| Baeten-2, 2015 [9] | Secukinumab 150 mg | 41.9±12.5 | 7.0±8.2 | 64 | 79 | 6.6±1.5 |
| Secukinumab 75 mg | 44.4±13.1 | 5.3±7.4 | 70 | 73 | 6.1±1.3 | |
| Placebo | 43.6±13.2 | 6.4±8.9 | 76 | 78 | 6.8±1.3 | |
| Huang, 2014 [10] | Adalimumab 40 mg | 30.1±8.7 | 3.0±3.8 | 80.8 | 95.6 | 6.0±1.4 |
| Placebo | 29.6±7.5 | 3.0±3.2 | 82.6 | 94.8 | 6.2±1.4 | |
| Lambert, 2007 [11] | Adalimumab 40 mg | 41.9±11.1 | 14.5±9.0 | 76.3 | 86.8 | 6.2±1.7 |
| Placebo | 40.0±10.9 | 12.1±8.7 | 81.8 | 81.8 | 6.5±1.6 | |
| van der Heijde, 2006 [12] | Adalimumab 40 mg | 41.7±11.9 | 11.3±9.99 | 75.5 | 78.4 | 6.3±1.7 |
| Placebo | 43.4±11.3 | 10.8±8.34 | 73.8 | 79.4 | 6.3±1.7 |
Table 3.
Characteristics of individual studies included in the network meta-analysis
| Comparison | Study | Patient |
|---|---|---|
| Placebo | 6 | 538 |
| Secukinumab 150 mg | 3 | 271 |
| Secukinumab 75 mg | 2 | 199 |
| Adalimumab 40 mg | 3 | 475 |
Table 4.
Network meta-analyses comprising the effects for all contrasts along with ORs and 95% credible intervals
Table 5.
Rank probability in terms of efficacy based on the number of patients that achieved an ASAS20 or ASAS40 response, and the safety based on the number of serious adverse events




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download