Abstract
Objective
Rebampide is a gastroprotective agent used to treat gastritis. It possesses anti-inflammatory and anti-arthritis effects, but the mechanisms of these effects are not well understood. The objective of this study was to explore mechanisms underlying the therapeutic effects of rebamipide in inflammatory arthritis.
Methods
Collagen-induced arthritis (CIA) was induced in DBA/1J mice. DBA/1J mice were immunized with chicken type II collagen, then treated intraperitoneally with rebamipide (10 mg/kg or 30 mg/kg) or vehicle (10% carboxymethylcellulose solution) alone. Seven weeks later, plasma samples were collected. Plasma metabolic profiles were analyzed using ultra performance liquid chromatography/quadrupole time-of-flight mass spectrometry-based metabolomics study and metabolite biomarkers were identified through multivariate data analysis.
Results
Low dose rebamipide treatment reduced the clinical arthritis score compared with vehicle treatment, whereas high dose rebamipide in CIA aggravated arthritis severity. Based on multivariate analysis, 17 metabolites were identified. The plasma levels of metabolites associated with fatty acids and phospholipid metabolism were significantly lower with rebamipide treatment than with vehicle. The levels of 15-deoxy-Δ12,14 prostaglandin J2 and thromboxane B3 decreased only in high dose-treated groups. Certain peptide molecules, including enterostatin (VPDPR) enterostatin and bradykinin dramatically increased in re-bamipide-treated groups at both doses. Additionally, corticosterone increased in the low dose-treated group and decreased in the high dose-treated group.
REFERENCES
1. Kobayashi T, Zinchuk VS, Garcia del Saz E, Jiang F, Yamasaki Y, Kataoka S, et al. Suppressive effect of rebamipide, an antiulcer agent, against activation of human neutrophils exposed to formyl-methionyl-leucyl-pheny-lalanine. Histol Histopathol. 2000; 15:1067–76.
2. Remans PH, Wijbrandts CA, Sanders ME, Toes RE, Breedveld FC, Tak PP, et al. CTLA-4IG suppresses reactive oxygen species by preventing synovial adherent cell-induced inactivation of Rap1, a Ras family GTPASE mediator of oxidative stress in rheumatoid arthritis T cells. Arthritis Rheum. 2006; 54:3135–43.

3. Kurimoto C, Kawano S, Tsuji G, Hatachi S, Jikimoto T, Sugiyama D, et al. Thioredoxin may exert a protective effect against tissue damage caused by oxidative stress in salivary glands of patients with Sjögren's syndrome. J Rheumatol. 2007; 34:2035–43.
4. Najim RA, Sharquie KE, Abu-Raghif AR. Oxidative stress in patients with Behcet's disease: I correlation with severity and clinical parameters. J Dermatol. 2007; 34:308–14.

5. Scott JL, Gabrielides C, Davidson RK, Swingler TE, Clark IM, Wallis GA, et al. Superoxide dismutase downregulation in osteoarthritis progression and end-stage disease. Ann Rheum Dis. 2010; 69:1502–10.

6. Kohashi M, Ishimaru N, Arakaki R, Hayashi Y. Effective treatment with oral administration of rebamipide in a mouse model of Sjögren's syndrome. Arthritis Rheum. 2008; 58:389–400.

7. Arimoto A, Kitagawa K, Mita N, Takahashi Y, Shibuya E, Sasaki H. Effect of rebamipide ophthalmic suspension on signs and symptoms of keratoconjunctivitis sicca in Sjögren syndrome patients with or without punctal occlusions. Cornea. 2014; 33:806–11.

8. Moon SJ, Park JS, Woo YJ, Lim MA, Kim SM, Lee SY, et al. Rebamipide suppresses collagen-induced arthritis through reciprocal regulation of th17/treg cell differentiation and heme oxygenase 1 induction. Arthritis Rheumatol. 2014; 66:874–85.

9. Moon SJ, Woo YJ, Jeong JH, Park MK, Oh HJ, Park JS, et al. Rebamipide attenuates pain severity and cartilage degeneration in a rat model of osteoarthritis by downregulating oxidative damage and catabolic activity in chondrocytes. Osteoarthritis Cartilage. 2012; 20:1426–38.

10. Visconti R, Grieco D. New insights on oxidative stress in cancer. Curr Opin Drug Discov Devel. 2009; 12:240–5.
11. Maritim AC, Sanders RA, Watkins JB 3rd. Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003; 17:24–38.

12. Rauscher FM, Sanders RA, Watkins JB 3rd. Effects of coen-zyme Q10 treatment on antioxidant pathways in normal and streptozotocin-induced diabetic rats. J Biochem Mol Toxicol. 2001; 15:41–6.

13. Surh YJ, Kundu JK, Na HK, Lee JS. Redox-sensitive transcription factors as prime targets for chemoprevention with anti-inflammatory and antioxidative phytochemicals. J Nutr. 2005; 135(12 Suppl):2993S–3001S.

14. Cortés-Jofré M, Rueda JR, Corsini-Muñoz G, Fonseca-Cortés C, Caraballoso M, Bonfill Cosp X. Drugs for preventing lung cancer in healthy people. Cochrane Database Syst Rev. 2012; 10:CD002141.

15. de Groot RH, Ouwehand C, Jolles J. Eating the right amount of fish: inverted U-shape association between fish consumption and cognitive performance and academic achievement in Dutch adolescents. Prostaglandins Leukot Essent Fatty Acids. 2012; 86:113–7.

16. Wang X, Zhang A, Sun H. Future perspectives of Chinese medical formulae: chinmedomics as an effector. OMICS. 2012; 16:414–21.

17. Rosloniec EF, Cremer M, Kang AH, Myers LK, Brand DD. Collagen-induced arthritis. Curr Protoc Immunol. 2010; Chapter 15: Unit 15.5.1-25.

18. Sangster T, Major H, Plumb R, Wilson AJ, Wilson ID. A pragmatic and readily implemented quality control strategy for HPLC-MS and GC-MS-based metabonomic analysis. Analyst. 2006; 131:1075–8.

19. Gika HG, Theodoridis GA, Wilson ID. Liquid chromatography and ultra-performance liquid chromatography-mass spectrometry fingerprinting of human urine: sample stability under different handling and storage conditions for metabonomics studies. J Chromatogr A. 2008; 1189; 314–22.
20. Lai L, Michopoulos F, Gika H, Theodoridis G, Wilkinson RW, Odedra R, et al. Methodological considerations in the development of HPLC-MS methods for the analysis of rodent plasma for metabonomic studies. Mol Biosyst. 2010; 6:108–20.

21. Xing J, Chen X, Sun Y, Luan Y, Zhong D. Interaction of bai-calin and baicalein with antibiotics in the gastrointestinal tract. J Pharm Pharmacol. 2005; 57:743–50.

22. Wu X, Cao H, Zhao L, Song J, She Y, Feng Y. Metabolomic analysis of glycerophospholipid signatures of inflammation treated with non-steroidal anti-inflammatory drugs-induced-RAW264.7 cells using (1)H NMR and U-HPLC/ Q-TOF-MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2016; 1028; 199–215.
24. Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids–new mechanisms for old drugs. N Engl J Med. 2005; 353:1711–23.
25. Straus DS, Glass CK. Cyclopentenone prostaglandins: new insights on biological activities and cellular targets. Med Res Rev. 2001; 21:185–210.

26. Bell-Parikh LC, Ide T, Lawson JA, McNamara P, Reilly M, FitzGerald GA. Biosynthesis of 15-deoxy-delta12,14-PGJ2 and the ligation of PPARgamma. J Clin Invest. 2003; 112:945–55.
27. Chawla A, Boisvert WA, Lee CH, Laffitte BA, Barak Y, Joseph SB, et al. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell. 2001; 7:161–71.
28. Ren D, Collingwood TN, Rebar EJ, Wolffe AP, Camp HS. PPARgamma knockdown by engineered transcription factors: exogenous PPARgamma2 but not PPARgamma1 re-activates adipogenesis. Genes Dev. 2002; 16:27–32.
29. Shi MA, Shi GP. Different roles of mast cells in obesity and diabetes: lessons from experimental animals and humans. Front Immunol. 2012; 3:7.

30. Jessop DS, Harbuz MS. A defect in cortisol production in rheumatoid arthritis: why are we still looking? Rheumatology (Oxford). 2005; 44:1097–100.

31. Straub RH, Paimela L, Peltomaa R, Schölmerich J, Leirisalo-Repo M. Inadequately low serum levels of steroid hormones in relation to interleukin-6 and tumor necrosis factor in untreated patients with early rheumatoid arthritis and reactive arthritis. Arthritis Rheum. 2002; 46:654–62.

32. Straub RH, Buttgereit F, Cutolo M. Alterations of the hypo-thalamic-pituitary-adrenal axis in systemic immune diseases – a role for misguided energy regulation. Clin Exp Rheumatol. 2011; 29(5 Suppl 68):S23–31.
33. Tsigos C, Papanicolaou DA, Defensor R, Mitsiadis CS, Kyrou I, Chrousos GP. Dose effects of recombinant human interleukin-6 on pituitary hormone secretion and energy expenditure. Neuroendocrinology. 1997; 66:54–62.
34. del Rey A, Wolff C, Wildmann J, Randolf A, Hahnel A, Besedovsky HO, Straub RH. Disrupted brain-immune system-joint communication during experimental arthritis. Arthritis Rheum. 2008; 58:3090–9.

35. Spurney RF, Fan PY, Ruiz P, Sanfilippo F, Pisetsky DS, Coffman TM. Thromboxane receptor blockade reduces renal injury in murine lupus nephritis. Kidney Int. 1992; 41:973–82.

36. Perico N, Rossini M, Imberti O, Malanchini B, Cornejo RP, Gaspari F, et al. Thromboxane receptor blockade attenuates chronic cyclosporine nephrotoxicity and improves survival in rats with renal isograft. J Am Soc Nephrol. 1992; 2:1398–404.

37. Smith SR, Creech EA, Schaffer AV, Martin LL, Rakhit A, Douglas FL, et al. Effects of thromboxane synthase inhibition with CGS 13080 in human cyclosporine nephrotoxicity. Kidney Int. 1992; 41:199–205.

38. Lin L, Park M, York DA. Enterostatin inhibition of dietary fat intake is modulated through the melanocortin system. Peptides. 2007; 28:643–9.

Figure 1.
Treatment with rebamipide (10 mg/kg) suppresses inflammatory arthritis in mice with collagen-induced arthritis (CIA). CIA was induced in DBA/1J mice by immunization with type II collagen (CII) in adjuvant. Changes in arthritis score in rebamipide-treated mice compared with vehicle-treated mice. Rebamipide dissolved in 10% carboxymethylcellulose solution (vehicle) was given intraperitoneally to 2 different groups (each receiving 10 or 30 mg/kg; n=10 mice per group) daily for 4 weeks, starting after booster immunization. A third group (n=10) received vehicle alone. WT: wild-type, D: day.
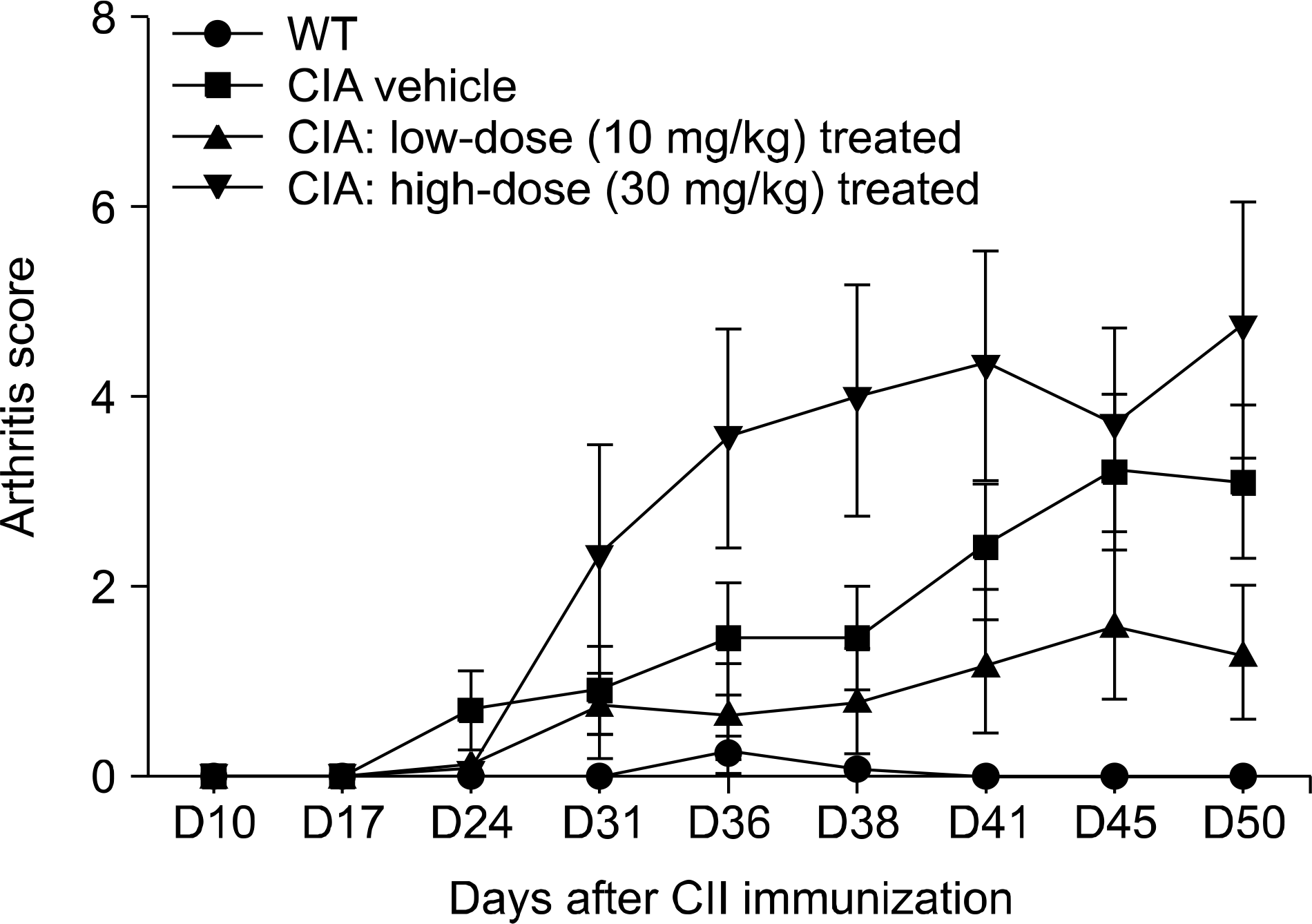
Figure 2.
(A) Principal component analysis (PCA) score plots and (B) partial least square-discriminant analysis (PLS-DA) score plots based on plasma metabolic profiling. (a) Positive ionization mode. (b) Negative ionization mode. In PCA, the score plot was obtained with the two PCs presenting 47.2% (PC1) and 14.5% (PC2) variance in positive ionization mode and that was with three PCs presenting 23.3% (PC1), 18.7% (PC2), and 13.2% (PC3) in negative ionization mode. In PLS-DA, the score plot was obtained with the two PCs presenting 43.2% (PC1) and 30.5% (PC2) variance in positive ionization mode and that was with three PCs presenting 53.7% (PC1), 38.6% (PC2), and 23.3% (PC3) in negative ionization mode.

Figure 3.
Comparison of metabolites with significant changes involved in (A) peptide metabolism, (B) fatty acid metabolism, (C) phospholipid metabolism, (D) acylcarnitine (β-oxidation) metabolism, (E) prostaglandin metabolism, and (F) corticosteroid hormone metabolism. *p<0.05, **p<0.01, and ***p<0.001 compared with vehicle control. HpODE: hydroxyoctadecadienoic acid, LysoPC: lysophosphatidylcholine, PGJ2: prostaglandin J2.
Table 1.
Identification of potential biomarkers
| rRT_m/z | Molecular formula | Fragmentation | Identified metabolite | Related metabolism |
Change trend |
|||
|---|---|---|---|---|---|---|---|---|
| W vs. V | L vs. V | H vs. V | H vs. L | |||||
| 0.34_317.18 | C23 H40 N8 O6 | C17 H17 N3 O2 | Enterostatin (VPDPR) | Peptide | – | ↑∗ | ↑∗ | – |
| [M+H] | (− C16 H24 N5 O4) | |||||||
| 1.04_354.19 | C50 H73 N15 O11 | C32 H50 N10 O7 | Bradykinin | – | ↑∗ | ↑∗ | – | |
| [M+H] | (− C18 H24 N5 O4) | |||||||
| 1.94_305.25 | C20 H32 O2 | C17 H25 | Arachidonic acid | Fatty acid | – | ↓ | ↓ | – |
| [M+H] | (− C3 H6 O2) | |||||||
| 2.03_257.25 | C16 H32 O2 | C16 H33 O | Palmitic acid | – | ↓∗ | ↓∗ | – | |
| [M+H] | (− O) | |||||||
| 2.07_283.27 | C18 H34 O2 | C17 H35 | Oleic acid | – | ↓ | ↓ | – | |
| [M+H] | (− CO2) | |||||||
| 1.51_333.20 | C18 H34 O5 | C17 H35 O3 | HpODE | – | ↓ | ↓ | – | |
| [M+Na-2H] | (− CO2) | |||||||
| 1.56_468.31 | C22 H46 NO7 P | C5 H13 O5 P | LysoPC (14:0) | Phospholipid | – | ↓∗ | ↓∗ | – |
| [M+H] | (− C17 H34 NO2) | |||||||
| 1.80_496.34 | C24 H50 NO7 P | C5 H13 O5 P | LysoPC (16:0) | – | ↓∗ | ↓∗ | – | |
| [M+H] | (− C19 H38 NO2) | |||||||
| 1.99_524.37 | C26 H54 NO7 P | C5 H13 O5 P | LysoPC (18:0) | – | ↓∗ | ↓∗ | – | |
| [M+H] | (− C21 H42 NO2) | |||||||
| 2.77_787.67 | C44 H86 NO8 P | C5 H13 O5 P | PC (18:0/18:1) | – | ↓ | – | – | |
| [M+H] | (− C39 H74 NO3) | |||||||
| 1.25-370.30 | C21 H39 NO4 | C8 H14 O3 | Tetradecenoylcarnitine | Acylcarnitine | – | ↓∗ | ↓∗ | – |
| [M+H] | (− C13 H26 NO) | (β-oxidation) | ||||||
| 1.34_372.31 | C21 H41 NO4 | C8 H14 O3 | Tetradecanoylcarnitine | – | ↓∗ | ↓∗ | – | |
| [M+H] | (− C13 H28 NO) | |||||||
| 1.40_398.33 | C23 H43 NO4 | C8 H14 O3 | Hexadecenoylcarnitine | – | ↓∗ | ↓∗ | – | |
| [M+H] | (− C15 H30 NO) | |||||||
| 1.53_400.43 | C23 H45 NO4 | C8 H14 O3 | Palmitoylcarnitine | – | ↓∗ | ↓∗ | ↓∗ | |
| [M+H] | (− C15 H32 NO) | |||||||
| 1.84_317.18 | C20 H30 O4 | C12 H16 O3 | 15-deoxy-Δ12,14 PGJ2 | Prostaglandin | – | – | ↓ | ↓∗ |
| [M+H] | (− C8 H15 O) | |||||||
| 2.21_369.23 | C20 H32 O6 | C12 H17 O5 | Thromboxane B3 | – | – | ↓∗ | ↓ | |
| [M+H] | (− C8 H15 O) | |||||||
| 2.07_381.17 | C21 H30 O4 | C21 H28 O3 | Corticosterone | Corticosteroid | – | ↑∗ | ↓ | ↓∗ |
| [M+Cl] | (− H2 O) | hormone | ||||||
rRT=retention time of metabolite/retention time of internal standard (IS) (reserpine). ↓: indicates decrease, ↑: indicates increase,–: indicates no significant changes, W: wild type, V: vehicle-treated CIA mice, L: low-dose rebamipide (10 mg/kg)-treated CIA mice, H: high-dose rebamipide (30 mg/kg)-treated CIA mice, HpODE: hydroxyoctadecadienoic acid, LysoPC: lysophosphatidylcholine, PGJ2: prostaglandin J2.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download