Abstract
Most rheumatic diseases are chronic inflammatory diseases. Kidney-related symptoms of rheumatic diseases are often present, which increase mortality and morbidity of patients with rheumatic diseases. When patients with rheumatic diseases show signs or symptoms of renal involvement, management for primary rheumatic diseases should be more aggressive. In general, the risk and severity of renal involvement in patients with rheumatic diseases depend on the type of primary rheumatic diseases. Rheumatic disease itself, chronic use of immunosuppressive agents and nonsteroidal anti-inflammatory drugs, and comorbidities, such as diabetes, hypertension, and cardiovascular complications, are the main causes of renal involvement in patients with rheumatic diseases. Many studies have reported the predominant features of renal involvement in most rheumatic diseases. We have attempted to summarize the relationships between rheumatic diseases and renal diseases, and clinical or pathophysiological features of renal involvement resulting from primary rheumatic diseases except systemic lupus erythematosus. Review for renal involvement, particularly in relation to early diagnosis and management of renal involvement in rheumatic diseases, is clinically significant because renal involvement in rheumatic diseases generally implies a bad prognosis.
Go to : 
REFERENCES
1. Chiu HY, Huang HL, Li CH, Chen HA, Yeh CL, Chiu SH, et al. Increased risk of chronic kidney disease in rheumatoid arthritis associated with cardiovascular complications – a national population-based cohort study. PLoS One. 2015; 10:e0136508.

3. Gilani ST, Khan DA, Khan FA, Ahmed M. Adverse effects of low dose methotrexate in rheumatoid arthritis patients. J Coll Physicians Surg Pak. 2012; 22:101–4.
4. Manabe S, Banno M, Nakano M, Fujii T, Fujiwara M, Kita Y, et al. Bucillamine-induced membranous nephropathy with crescent formation in a patient with rheumatoid arthritis: case report and literature review. Case Rep Nephrol Dial. 2014; 5:30–8.

5. Möller B, Pruijm M, Adler S, Scherer A, Villiger PM, Finckh A. Swiss Clinical Quality Management in Rheumatic Diseases (SCQM) Foundation, CH-8048 Zurich, Switzerland. Chronic NSAID use and long-term decline of renal function in a prospective rheumatoid arthritis cohort study. Ann Rheum Dis. 2015; 74:718–23.

6. Kaushik P, Rahmani M, Ellison W. Membranous glomerulonephritis with the use of etanercept in ankylosing spondylitis. Ann Pharmacother. 2011; 45:e62.

7. Tokoroyama T, Ando M, Setoguchi K, Tsuchiya K, Nitta K. Prevalence, incidence and prognosis of chronic kidney disease classified according to current guidelines: a large retrospective cohort study of rheumatoid arthritis patients. Nephrol Dial Transplant. 2016 Sep 16; [Epub].DOI: DOI: 10.1093/ndt/gfw315.

8. Mittal T, Rathi M. Rheumatological diseases and kidneys: a nephrologist's perspective. Int J Rheum Dis. 2014; 17:834–44.

10. Carmona L, Cross M, Williams B, Lassere M, March L. Rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2010; 24:733–45.

11. Vinicki JP, Pellet SC, De Rosa G, Dubinsky D, Laborde HA, Marini A, et al. Analysis of 65 renal biopsies from patients with rheumatoid arthritis (1976-2015): Change in treatment strategies decreased frequency and modified histopathological findings. J Clin Rheumatol. 2015; 21:335–40.
12. Makino H, Yoshinaga Y, Yamasaki Y, Morita Y, Hashimoto H, Yamamura M. Renal involvement in rheumatoid arthritis: analysis of renal biopsy specimens from 100 patients. Mod Rheumatol. 2002; 12:148–54.

13. Hickson LJ, Crowson CS, Gabriel SE, McCarthy JT, Matteson EL. Development of reduced kidney function in rheumatoid arthritis. Am J Kidney Dis. 2014; 63:206–13.

14. Naranjo A, Sokka T, Descalzo MA, Calvo-Alén J, Hørslev-Petersen K, Luukkainen RK, et al. Cardiovascular disease in patients with rheumatoid arthritis: results from the QUEST-RA study. Arthritis Res Ther. 2008; 10:R30.

15. Helin HJ, Korpela MM, Mustonen JT, Pasternack AI. Renal biopsy findings and clinicopathologic correlations in rheumatoid arthritis. Arthritis Rheum. 1995; 38:242–7.

16. Icardi A, Araghi P, Ciabattoni M, Romano U, Lazzarini P, Bianchi G. Kidney involvement in rheumatoid arthritis. Reumatismo. 2003; 55:76–85.

17. Karie S, Gandjbakhch F, Janus N, Launay-Vacher V, Rozenberg S, Mai Ba CU, et al. Kidney disease in RA patients: prevalence and implication on RA-related drugs management: the MATRIX study. Rheumatology (Oxford). 2008; 47:350–4.

18. Ramos-Casals M, Brito-Zerón P, Sisó-Almirall A, Bosch X. Primary Sjogren syndrome. BMJ. 2012; 344:e3821.

19. Talal N, Zisman E, Schur PH. Renal tubular acidosis, glomerulonephritis and immunologic factors in Sjögren's syndrome. Arthritis Rheum. 1968; 11:774–86.
20. François H, Mariette X. Renal involvement in primary Sjögren syndrome. Nat Rev Nephrol. 2016; 12:82–93.

21. Maripuri S, Grande JP, Osborn TG, Fervenza FC, Matteson EL, Donadio JV, et al. Renal involvement in primary Sjögren's syndrome: a clinicopathologic study. Clin J Am Soc Nephrol. 2009; 4:1423–31.

22. Bossini N, Savoldi S, Franceschini F, Mombelloni S, Baronio M, Cavazzana I, et al. Clinical and morphological features of kidney involvement in primary Sjögren's syndrome. Nephrol Dial Transplant. 2001; 16:2328–36.

23. Goules A, Masouridi S, Tzioufas AG, Ioannidis JP, Skopouli FN, Moutsopoulos HM. Clinically significant and biop-sy-documented renal involvement in primary Sjögren syndrome. Medicine (Baltimore). 2000; 79:241–9.
24. Ren H, Wang WM, Chen XN, Zhang W, Pan XX, Wang XL, et al. Renal involvement and followup of 130 patients with primary Sjögren's syndrome. J Rheumatol. 2008; 35:278–84.
25. Baldini C, Pepe P, Quartuccio L, Priori R, Bartoloni E, Alunno A, et al. Primary Sjogren's syndrome as a multiorgan disease: impact of the serological profile on the clinical presentation of the disease in a large cohort of Italian patients. Rheumatology (Oxford). 2014; 53:839–44.
26. Evan AP, Lingeman J, Coe F, Shao Y, Miller N, Matlaga B, et al. Renal histopathology of stone-forming patients with distal renal tubular acidosis. Kidney Int. 2007; 71:795–801.

27. Bonny O, Rubin A, Huang CL, Frawley WH, Pak CY, Moe OW. Mechanism of urinary calcium regulation by urinary magnesium and pH. J Am Soc Nephrol. 2008; 19:1530–7.

28. Falk RJ, Hogan S, Carey TS, Jennette JC. Clinical course of anti-neutrophil cytoplasmic autoantibody-associated glomerulonephritis and systemic vasculitis. The Glomerular Disease Collaborative Network. Ann Intern Med. 1990; 113:656–63.
29. Furuta S, Jayne DR. Antineutrophil cytoplasm antibody-associated vasculitis: recent developments. Kidney Int. 2013; 84:244–9.

30. Jennette JC. Rapidly progressive crescentic glomerulonephritis. Kidney Int. 2003; 63:1164–77.
31. Kim HW, Song YW. ANCA-associated vasculitis: report from Korea. Clin Exp Nephrol. 2013; 17:708–11.

32. Johnson RJ, Feehally J, Floege J. Comprehensive clinical nephrology. 5th ed.Philadelphia: Saunders Press;2014. p. 291–6.
33. Chen YX, Xu J, Pan XX, Shen PY, Li X, Ren H, et al. Histopathological classification and renal outcome in patients with antineutrophil cytoplasmic antibodies-associated renal vasculitis: a study of 186 patients and metaanalysis. J Rheumatol. 2017; 44:304–13.

34. Berden AE, Ferrario F, Hagen EC, Jayne DR, Jennette JC, Joh K, et al. Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol. 2010; 21:1628–36.

35. Abdulahad WH, Lamprecht P, Kallenberg CG. T-helper cells as new players in ANCA-associated vasculitides. Arthritis Res Ther. 2011; 13:236.

36. Xing GQ, Chen M, Liu G, Heeringa P, Zhang JJ, Zheng X, et al. Complement activation is involved in renal damage in human antineutrophil cytoplasmic autoantibody associated pauci-immune vasculitis. J Clin Immunol. 2009; 29:282–91.

37. Cornec D, Cornec-Le Gall E, Fervenza FC, Specks U. ANCA-associated vasculitis – clinical utility of using ANCA specificity to classify patients. Nat Rev Rheumatol. 2016; 12:570–9.

38. Harper L, Morgan MD, Walsh M, Hoglund P, Westman K, Flossmann O, et al. Pulse versus daily oral cyclophosphamide for induction of remission in ANCA-associated vasculitis: long-term follow-up. Ann Rheum Dis. 2012; 71:955–60.

39. Klemmer PJ, Chalermskulrat W, Reif MS, Hogan SL, Henke DC, Falk RJ. Plasmapheresis therapy for diffuse alveolar hemorrhage in patients with small-vessel vasculitis. Am J Kidney Dis. 2003; 42:1149–53.

40. Jayne DR, Gaskin G, Rasmussen N, Abramowicz D, Ferrario F, Guillevin L, et al. European Vasculitis Study Group. Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol. 2007; 18:2180–8.

41. Walsh M, Catapano F, Szpirt W, Thorlund K, Bruchfeld A, Guillevin L, et al. Plasma exchange for renal vasculitis and idiopathic rapidly progressive glomerulonephritis: a meta-analysis. Am J Kidney Dis. 2011; 57:566–74.

42. Mouthon L, Bussone G, Berezné A, Noël LH, Guillevin L. Scleroderma renal crisis. J Rheumatol. 2014; 41:1040–8.

43. Ostojic P, Stojanovski N. Arterial hypertension treated with angiotensin converting enzyme inhibitors and glucocorticoids are independent risk factors associated with decreased glomerular filtration rate in systemic sclerosis. Rheumatol Int. 2017; 37:363–8.

44. Logee KM, Lakshminarayanan S. Scleroderma renal crisis as an initial presentation of systemic sclerosis: a case report and review of the literature. Clin Exp Rheumatol. 2015; 33(4 Suppl 91):S171–4.
45. Steen VD, Costantino JP, Shapiro AP, Medsger TA Jr. Outcome of renal crisis in systemic sclerosis: relation to availability of angiotensin converting enzyme (ACE) inhibitors. Ann Intern Med. 1990; 113:352–7.

46. Fisher ER, Rodnan GP. Pathologic observations concerning the kidney in progressive systemic sclerosis. AMA Arch Pathol. 1958; 65:29–39.
47. Kobayashi H, Nishimaki T, Kaise S, Suzuki T, Watanabe K, Kasukawa R, et al. Immunohistological study endothelin-1 and endothelin-A and B receptors in two patients with scleroderma renal crisis. Clin Rheumatol. 1999; 18:425–7.
48. Vancheeswaran R, Magoulas T, Efrat G, Wheeler-Jones C, Olsen I, Penny R, et al. Circulating endothelin-1 levels in systemic sclerosis subsets–a marker of fibrosis or vascular dysfunction? J Rheumatol. 1994; 21:1838–44.
49. Okano Y, Steen VD, Medsger TA Jr. Autoantibody reactive with RNA polymerase III in systemic sclerosis. Ann Intern Med. 1993; 119:1005–13.

50. Kang EH, Im CH, Kim SH, Chung JR, Lee EY, Kim DJ, et al. A case of renal crisis in a Korean scleroderma patient with anti-RNA polymerase I and III antibodies. J Korean Med Sci. 2006; 21:1121–3.

52. Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, et al. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005; 67:1739–42.

53. Avram Z, Krishnan E. Hyperuricaemia–where nephrology meets rheumatology. Rheumatology (Oxford). 2008; 47:960–4.

55. Latif W, Karaboyas A, Tong L, Winchester JF, Arrington CJ, Pisoni RL, et al. Uric acid levels and all-cause and cardiovascular mortality in the hemodialysis population. Clin J Am Soc Nephrol. 2011; 6:2470–7.

56. Zhu Y, Pandya BJ, Choi HK. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007-2008. Am J Med. 2012; 125:679–87.e1.

57. Faruque LI, Ehteshami-Afshar A, Wiebe N, Tjosvold L, Homik J, Tonelli M. A systematic review and meta-analysis on the safety and efficacy of febuxostat versus allopurinol in chronic gout. Semin Arthritis Rheum. 2013; 43:367–75.

58. Khanna D, Fitzgerald JD, Khanna PP, Bae S, Singh MK, Neogi T, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic non-pharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). 2012; 64:1431–46.

Go to : 
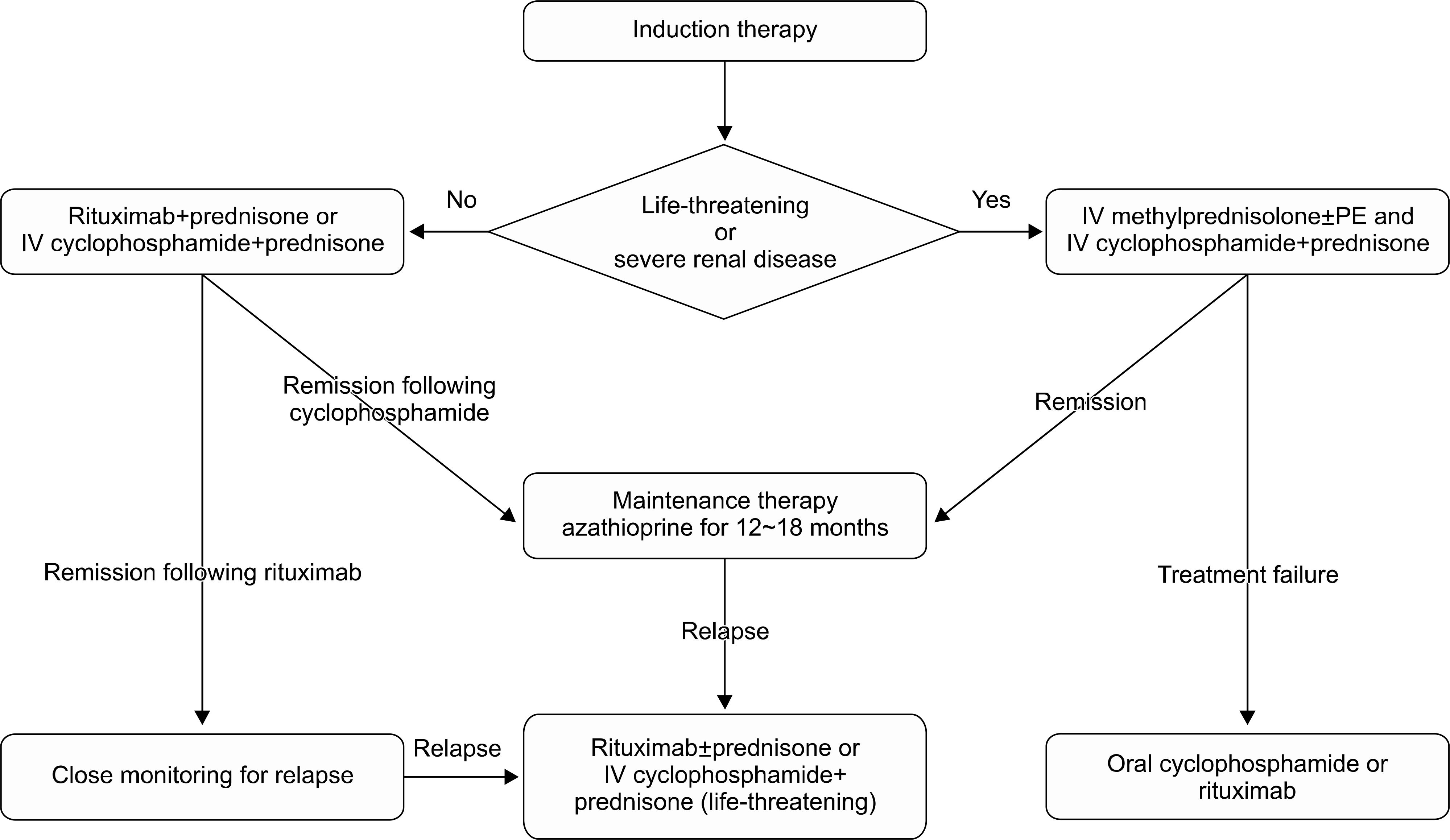 | Figure 1.Treatment of ANCA-associated renal vasculitis. IV methylprednisolone usually begins with 7 mg/kg per day of methylprednisolone for 3 days and followed by oral prednisone 1 mg/kg per day. IV cyclophosphamide usually begins with 0.5 g/m2 per monthly of cyclophosphamide. Oral cyclophosphamide usually begins with 2 mg/kg per day of cyclophosphamide and the dose can be reduced based on renal function or age. Oral prednisone should be tapered slowly during 3 to 6 months. Oral azathioprine usually begin with 2 mg/kg per day of azathioprine. ANCA: anti-neutrophilic cytoplasmic antibody, IV: intravenous, PE: plasma exchange. |
Table 1.
Characteristics of urinary dipstick test
Table 2.
Renal screening tests in patients with primary Sjögren's syndrome
Table 3.
Frequency of systemic involvement in ANCA-associated small vessel vasculitis
| Systemic organ |
Frequency of involvement |
||
|---|---|---|---|
| MPA | GPA | EGPA | |
| Kidney | 90 | 80 | 45 |
| Skin | 40 | 40 | 50 |
| Lung | 50 | 90 | 90 |
| ENT | 35 | 90 | 50 |
| Musculoskeletal | 60 | 60 | 50 |
| Neurologic | 30 | 50 | 60 |
Table 4.
Classification for ANCA-associated glomerulonephritis
Table 5.
Several randomized controlled trials (RCTs) in treatment of ANCA-associated vasculitis




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download