Abstract
Interleukin-32 (IL-32), a recently identified pro-inflammatory cytokine, is involved in the pathogenesis and progression of infections, cancer, chronic inflammation, and autoimmune disease. IL-32γ is the most active isoform in cell death and cell activation among nine distinct isoforms of IL-32. IL-32γ potentiates both osteogenic and osteoclastogenic capacities, and is critical in the coupling of bone resorption and bone formation for maintenance of bone homeostasis. IL-32γ is strongly associated with inflammatory bone disorders such as rheumatoid arthritis, ankylosing spondylitis, and osteoporosis. In this review, we summarize current research on the role of IL-32γ in inflammatory bone disorders, highlighting this cytokine as a novel target for prognostic marker and control of these diseases.
Go to : 
REFERENCES
1. McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007; 7:429–42.

2. Sveaas SH, Berg IJ, Provan SA, Semb AG, Olsen IC, Ueland T, et al. Circulating levels of inflammatory cytokines and cytokine receptors in patients with ankylosing spondylitis: a cross-sectional comparative study. Scand J Rheumatol. 2015; 44:118–24.

3. Lee EJ, Kim SM, Choi B, Kim EY, Chung YH, Lee EJ, et al. Interleukin-32 gamma stimulates bone formation by increasing miR-29a in osteoblastic cells and prevents the development of osteoporosis. Sci Rep. 2017; 7:40240.

4. Lee EJ, Lee EJ, Chung YH, Song DH, Hong S, Lee CK, et al. High level of interleukin-32 gamma in the joint of ankylosing spondylitis is associated with osteoblast differentiation. Arthritis Res Ther. 2015; 17:350.

5. Kim YG, Lee CK, Oh JS, Kim SH, Kim KA, Yoo B. Effect of interleukin-32gamma on differentiation of osteoclasts from CD14+ monocytes. Arthritis Rheum. 2010; 62:515–23.
6. Dahl CA, Schall RP, He HL, Cairns JS. Identification of a novel gene expressed in activated natural killer cells and T cells. J Immunol. 1992; 148:597–603.
7. Khawar B, Abbasi MH, Sheikh N. A panoramic spectrum of complex interplay between the immune system and IL-32 during pathogenesis of various systemic infections and inflammation. Eur J Med Res. 2015; 20:7.

8. Dinarello CA, Kim SH. IL-32, a novel cytokine with a possible role in disease. Ann Rheum Dis. 2006; 65(Suppl 3):iii61–4.

9. Khawar MB, Abbasi MH, Sheikh N. IL-32: A novel pluripotent inflammatory interleukin, towards gastric inflammation, gastric cancer, and chronic rhino sinusitis. Mediators Inflamm. 2016; 2016; 8413768.

11. Xu WD, Zhang M, Feng CC, Yang XK, Pan HF, Ye DQ. IL-32 with potential insights into rheumatoid arthritis. Clin Immunol. 2013; 147:89–94.

12. Kim SH, Han SY, Azam T, Yoon DY, Dinarello CA. Interleu-kin-32: a cytokine and inducer of TNFalpha. Immunity. 2005; 22:131–42.
13. Calabrese F, Baraldo S, Bazzan E, Lunardi F, Rea F, Maestrelli P, et al. IL-32, a novel proinflammatory cytokine in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008; 178:894–901.

14. Shoda H, Fujio K, Yamaguchi Y, Okamoto A, Sawada T, Kochi Y, et al. Interactions between IL-32 and tumor necrosis factor alpha contribute to the exacerbation of immune-inflammatory diseases. Arthritis Res Ther. 2006; 8:R166.
15. Schenk M, Krutzik SR, Sieling PA, Lee DJ, Teles RM, Ochoa MT, et al. NOD2 triggers an interleukin-32-dependent human dendritic cell program in leprosy. Nat Med. 2012; 18:555–63.

16. Li W, Liu Y, Mukhtar MM, Gong R, Pan Y, Rasool ST, et al. Activation of interleukin-32 proinflammatory pathway in response to influenza A virus infection. PLoS One. 2008; 3:e1985.

17. Joosten LA, Netea MG, Kim SH, Yoon DY, Oppers-Walgreen B, Radstake TR, et al. IL-32, a proinflammatory cytokine in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2006; 103:3298–303.

18. Gui M, Zhang H, Zhong K, Li Y, Sun J, Wang L. Clinical significance of interleukin-32 expression in patients with rheumatoid arthritis. Asian Pac J Allergy Immunol. 2013; 31:73–8.
19. Kang JW, Park YS, Lee DH, Kim MS, Bak Y, Ham SY, et al. Interaction network mapping among IL-32 isoforms. Biochimie. 2014; 101:248–51.

20. Choi JD, Bae SY, Hong JW, Azam T, Dinarello CA, Her E, et al. Identification of the most active interleukin-32 isoform. Immunology. 2009; 126:535–42.

21. Nam SY, Oh HA, Choi Y, Park KY, Kim HM, Jeong HJ. Inhibition of IL-32 signaling by bamboo salt decreases proinflammatory responses in cellular models of allergic rhinitis. J Med Food. 2014; 17:939–48.

22. Choi KY, Napper S, Mookherjee N. Human cathelicidin LL-37 and its derivative IG-19 regulate interleukin-32-induced inflammation. Immunology. 2014; 143:68–80.

23. Heinhuis B, Plantinga TS, Semango G, Küsters B, Netea MG, Dinarello CA, et al. Alternatively spliced isoforms of IL-32 differentially influence cell death pathways in cancer cell lines. Carcinogenesis. 2016; 37:197–205.

24. Heinhuis B, Koenders MI, van den Berg WB, Netea MG, Dinarello CA, Joosten LA. Interleukin 32 (IL-32) contains a typical α-helix bundle structure that resembles focal adhesion targeting region of focal adhesion kinase-1. J Biol Chem. 2012; 287:5733–43.

25. Hong J, Bae S, Kang Y, Yoon D, Bai X, Chan ED, et al. Suppressing IL-32 in monocytes impairs the induction of the proinflammatory cytokines TNFalpha and IL-1beta. Cytokine. 2010; 49:171–6.
26. Li W, Sun W, Liu L, Yang F, Li Y, Chen Y, et al. IL-32: a host proinflammatory factor against influenza viral replication is upregulated by aberrant epigenetic modifications during influenza A virus infection. J Immunol. 2010; 185:5056–65.

27. Nold MF, Nold-Petry CA, Pott GB, Zepp JA, Saavedra MT, Kim SH, et al. Endogenous IL-32 controls cytokine and HIV-1 production. J Immunol. 2008; 181:557–65.

28. Rasool ST, Tang H, Wu J, Li W, Mukhtar MM, Zhang J, et al. Increased level of IL-32 during human immunodeficiency virus infection suppresses HIV replication. Immunol Lett. 2008; 117:161–7.

29. Zepp JA, Nold-Petry CA, Dinarello CA, Nold MF. Protection from RNA and DNA viruses by IL-32. J Immunol. 2011; 186:4110–8.

30. Nold-Petry CA, Nold MF, Zepp JA, Kim SH, Voelkel NF, Dinarello CA. IL-32-dependent effects of IL-1beta on endothelial cell functions. Proc Natl Acad Sci U S A. 2009; 106:3883–8.
31. Netea MG, Azam T, Lewis EC, Joosten LA, Wang M, Langenberg D, et al. Mycobacterium tuberculosis induces interleukin-32 production through a caspase-1/IL-18/ interferon-gamma-dependent mechanism. PLoS Med. 2006; 3:e277.
32. Heinhuis B, Koenders MI, van de Loo FA, van Lent PL, Kim SH, Dinarello CA, et al. IL-32gamma and Streptococcus pyogenes cell wall fragments synergise for IL-1-dependent destructive arthritis via upregulation of TLR-2 and NOD2. Ann Rheum Dis. 2010; 69:1866–72.
33. Bai X, Shang S, Henao-Tamayo M, Basaraba RJ, Ovrutsky AR, Matsuda JL, et al. Human IL-32 expression protects mice against a hypervirulent strain of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2015; 112:5111–6.

34. Seo EH, Kang J, Kim KH, Cho MC, Lee S, Kim HJ, et al. Detection of expressed IL-32 in human stomach cancer using ELISA and immunostaining. J Microbiol Biotechnol. 2008; 18:1606–12.
35. Sorrentino C, Di Carlo E. Expression of IL-32 in human lung cancer is related to the histotype and metastatic phenotype. Am J Respir Crit Care Med. 2009; 180:769–79.

36. Nishida A, Andoh A, Inatomi O, Fujiyama Y. Interleukin-32 expression in the pancreas. J Biol Chem. 2009; 284:17868–76.

37. Kang YH, Park MY, Yoon DY, Han SR, Lee CI, Ji NY, et al. Dysregulation of overexpressed IL-32α in hepatocellular carcinoma suppresses cell growth and induces apoptosis through inactivation of NF-κ B and Bcl-2. Cancer Lett. 2012; 318:226–33.
38. Lee HJ, Liang ZL, Huang SM, Lim JS, Yoon DY, Lee HJ, et al. Overexpression of IL-32 is a novel prognostic factor in patients with localized clear cell renal cell carcinoma. Oncol Lett. 2012; 3:490–6.

39. Soyka MB, Treis A, Eiwegger T, Menz G, Zhang S, Holzmann D, et al. Regulation and expression of IL-32 in chronic rhinosinusitis. Allergy. 2012; 67:790–8.

40. Shioya M, Nishida A, Yagi Y, Ogawa A, Tsujikawa T, Kim-Mitsuyama S, et al. Epithelial overexpression of interleukin-32alpha in inflammatory bowel disease. Clin Exp Immunol. 2007; 149:480–6.
41. Mabilleau G, Sabokbar A. Interleukin-32 promotes osteoclast differentiation but not osteoclast activation. PLoS One. 2009; 4:e4173.

42. Moon YM, Yoon BY, Her YM, Oh HJ, Lee JS, Kim KW, et al. IL-32 and IL-17 interact and have the potential to aggravate osteoclastogenesis in rheumatoid arthritis. Arthritis Res Ther. 2012; 14:R246.

43. Mun SH, Kim JW, Nah SS, Ko NY, Lee JH, Kim JD, et al. Tumor necrosis factor alpha-induced interleukin-32 is positively regulated via the Syk/protein kinase Cdelta/JNK pathway in rheumatoid synovial fibroblasts. Arthritis Rheum. 2009; 60:678–85.
Go to : 
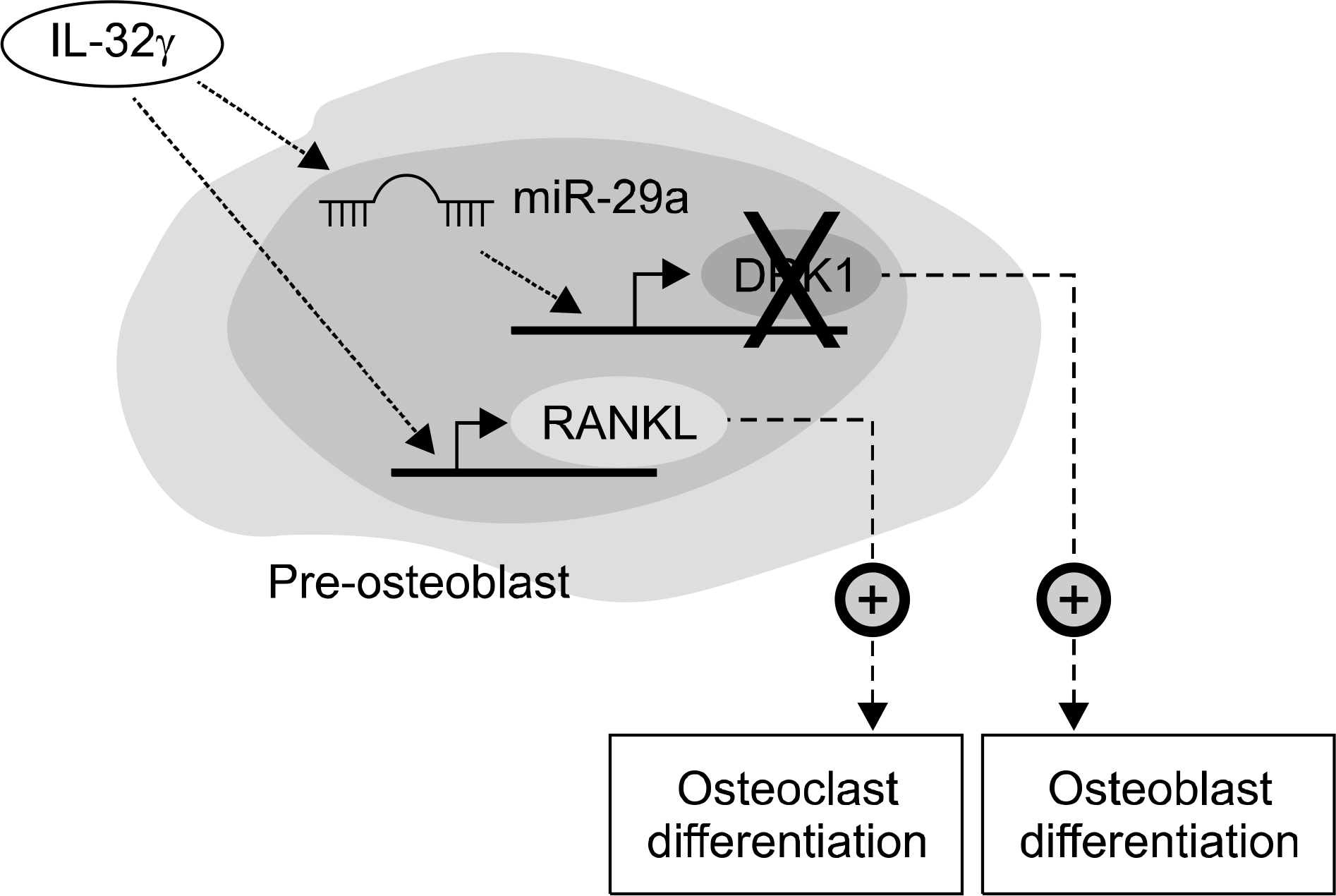 | Figure 1.Molecular mechanisms of interleukin (IL)-32γ-me-diated bone metabolism. IL-32γ increases the level of Dik-koprf-1 (DKK-1)-targeting miR-29a, leading to osteoblast differentiation and subsequent increase in bone formation. Simultaneously, IL-32γ enhances receptor activator of nuclear factor-kappa B ligand (RANKL) production to activate osteoclast differentiation. |
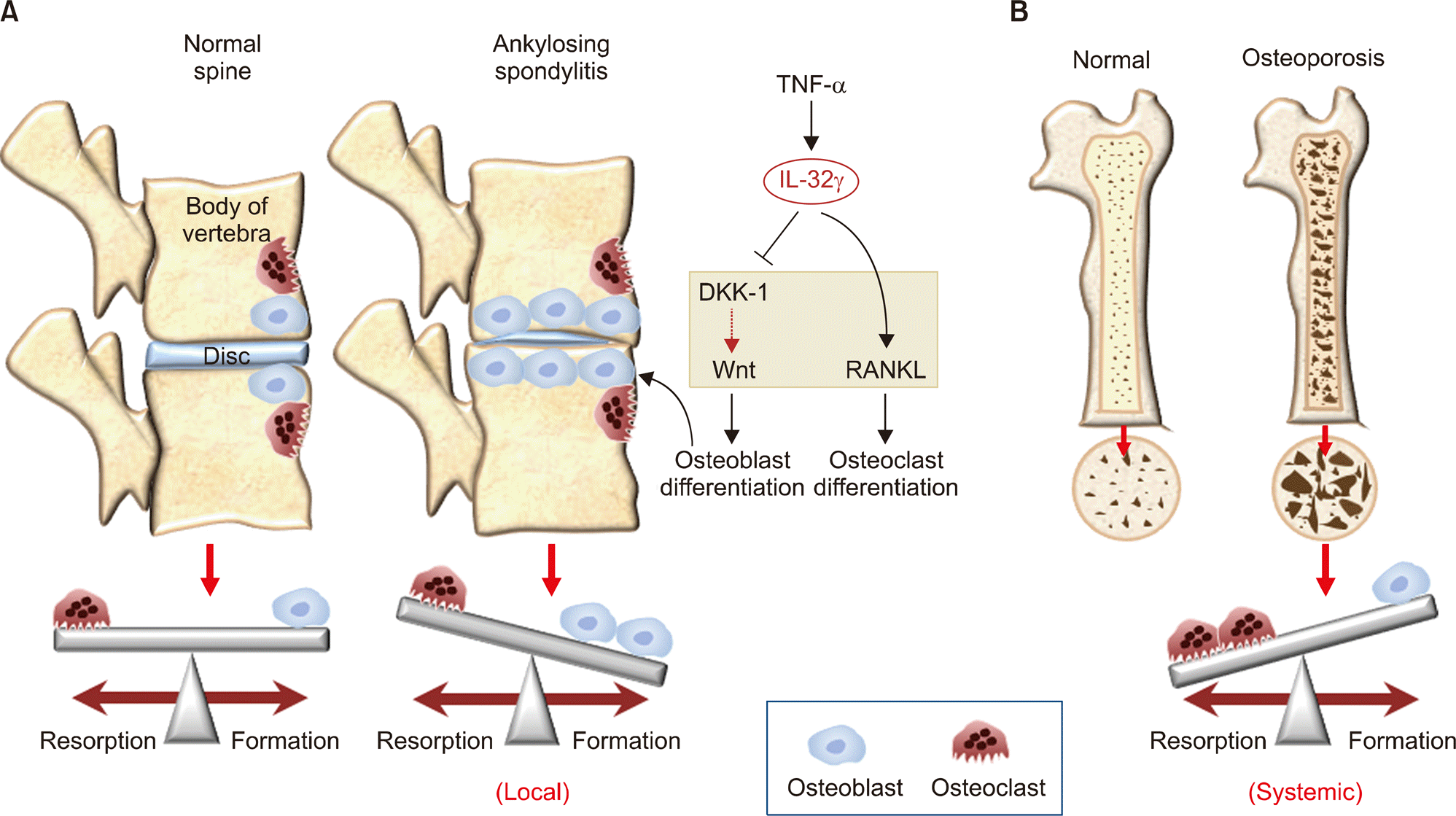 | Figure 2.The effects of interleukin (IL)-32γ on bone remodeling in ankylosing spondylitis and osteoporosis. (A) Normal spine remodeling is balanced by the interplay between bone-forming osteoblasts and bone-resorbing osteoclasts. In the spinal joints of patients with ankylosing spondylitis, locally elevated IL-32γ suppresses Dikkoprf-1 (DKK-1), a Wnt inhibitor in the synovium, which enables differentiation of osteoblast and subsequent new abnormal bone formation; this effect overshadows the effect of IL-32γ on receptor activator of nuclear factor-kappa B ligand (RANKL)-mediated osteoclast differentiation. (B) Diminished level of systemic IL-32γ in osteoporosis patients results in elevated expression of DKK-1, which leads to low bone mass and high fracture risk; however, there are no significant differences in bone marrow IL-32γ level between osteoporotic hip fracture patients and no-fracture patients. TNF-α: tumor necrosis factor-α. |
Table 1.
Diseases with known interleukin (IL)-32 involvement in pathogenicity




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download