Abstract
Hyperuricemia is related to metabolic syndrome, and is defined as an over-production or under-excretion of uric acid (UA), with increased UA serum concentration. Among other causes, Hyper-homocysteinemia (H-Hcy) can be responsible for hyperuricemia. The mechanisms underlying the association between these two conditions are unclear, but increased UA serum levels can be a consequence of renovascular atherosclerosis, with reduced UA excretion. An alternative hypothesis is the over-production of UA from adenosine (originating from S-adenosyl-homocysteine). Genetic polymorphism (C677T) of methylenetetrahydrofolate reductase (MTHFR) may contribute. A possible mechanism is purines biosyinthesis originating from this gene variant. However, the results obtained from several studies and meta-analyses of the relationship between H-Hcy and hyperuricemia are ambivalent, and broader research is needed.
Go to : 
REFERENCES
2. Eggebeen AT. Gout: an update. Am Fam Physician. 2007; 76:801–8.
3. Kuzuya M, Ando F, Iguchi A, Shimokata H. Effect of aging on serum uric acid levels: longitudinal changes in a large Japanese population group. J Gerontol A Biol Sci Med Sci. 2002; 57:M660–4.

4. Sumino H, Ichikawa S, Kanda T, Nakamura T, Sakamaki T. Reduction of serum uric acid by hormone replacement therapy in postmenopausal women with hyperuricaemia. Lancet. 1999; 354:650.

5. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011; 63:3136–41.

6. Desideri G, Puig JG, Richette P. The management of hyperuricemia with urate deposition. Curr Med Res Opin. 2015; 31(Suppl 2):27–32.

7. Meneses-Leon J, Denova-Gutiérrez E, Castañón-Robles S, Granados-García V, Talavera JO, Rivera-Paredez B, et al. Sweetened beverage consumption and the risk of hyperuricemia in Mexican adults: a cross-sectional study. BMC Public Health. 2014; 14:445.

8. Koenig W, Meisinger C. Uric acid, type 2 diabetes, and cardiovascular diseases: fueling the common soil hypothesis? Clin Chem. 2008; 54:231–3.

9. Li M, Hou W, Zhang X, Hu L, Tang Z. Hyperuricemia and risk of stroke: a systematic review and meta-analysis of prospective studies. Atherosclerosis. 2014; 232:265–70.

10. Kelley WN, Grobner W, Holmes E. Current concepts in the pathogenesis of hyperuricemia. Metabolism. 1973; 22:939–59.

11. Malinow MR, Levenson J, Giral P, Nieto FJ, Razavian M, Segond P, et al. Role of blood pressure, uric acid, and hemor-heological parameters on plasma homocyst(e)ine concentration. Atherosclerosis. 1995; 114:175–83.

12. Miller AL, Kelly GS. Homocysteine metabolism: nutritional modulation and impact on health and disease. Alt Med Rev. 1997; 2:234–54.
13. Cohen E, Levi A, Vecht-Lifshitz SE, Goldberg E, Garty M, Krause I. Assessment of a possible link between hyperhomocysteinemia and hyperuricemia. J Investig Med. 2015; 63:534–8.

14. Prasad Sah OS, Qing YX. Associations between hyperuricemia and chronic kidney disease: a review. Nephrourol Mon. 2015; 7:e27233.

15. Zhang S, Bai YY, Luo LM, Xiao WK, Wu HM, Ye P. Association between serum homocysteine and arterial stiffness in elderly: a community-based study. J Geriatr Cardiol. 2014; 11:32–8.
16. Ganguly P, Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutr J. 2015; [Epub].DOI: DOI: 10.1186/1475-2891-14-6.

17. Jamaluddin MD, Chen I, Yang F, Jiang X, Jan M, Liu X, et al. Homocysteine inhibits endothelial cell growth via DNA hypomethylation of the cyclin A gene. Blood. 2007; 110:3648–55.
18. Robinson K, Mayer E, Jacobsen DW. Homocysteine and coronary artery disease. Cleve Clin J Med. 1994; 61:438–50.

19. El-Khairy L, Ueland PM, Refsum H, Graham IM, Vollset SE. Plasma total cysteine as a risk factor for vascular disease: the European concerted action project. Circulation. 2001; 103:2544–9.
20. Lai S, Mariotti A, Coppola B, Lai C, Aceto P, Dimko M, et al. Uricemia and homocysteinemia: nontraditional risk factors in the early stages of chronic kidney disease–preliminary data. Eur Rev Med Pharmacol Sci. 2014; 18:1010–7.
21. Wei W, Liu SY, Zeng FF, Ma L, Li KS, Wang BY. Meta-analysis of the association of the C677T polymorphism of the methylenetetrahydrofolate reductase gene with hyperuricemia. Ann Nutr Metab. 2012; 60:44–51.

22. Zuo M, Nishio H, Lee MJ, Maejima K, Mimura S, Sumino K. The C677T mutation in the methylene tetrahydrofolate reductase gene increases serum uric acid in elderly men. J Hum Genet. 2000; 45:257–62.

23. Shi HY, Dong YN, Nan HR, Qian WW, Qian RL. Association of the methylenetretrahydrofolate reductase (MTHFR) gene C677T polymorphism and hyperuricemia. Chin J Diabetes. 2006; 14:178–81.
24. Golbahar J, Aminzadeh MA, Al-Shboul QM, Kassab S, Rezaian GR. Association of methylenetetrahydrofolate reductase (C677T) polymorphism with hyperuricemia. Nutr Metab Cardiovasc Dis. 2007; 17:462–7.

25. Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995; 10:111–3.

26. Rai V. The MTHFR C677T polymorphism and hyperuricemia risk: a meta-analysis of 558 cases and 912 controls. Metabolomics. 2016; [Epub].DOI: DOI: 10.4172/2153-0769.1000166.

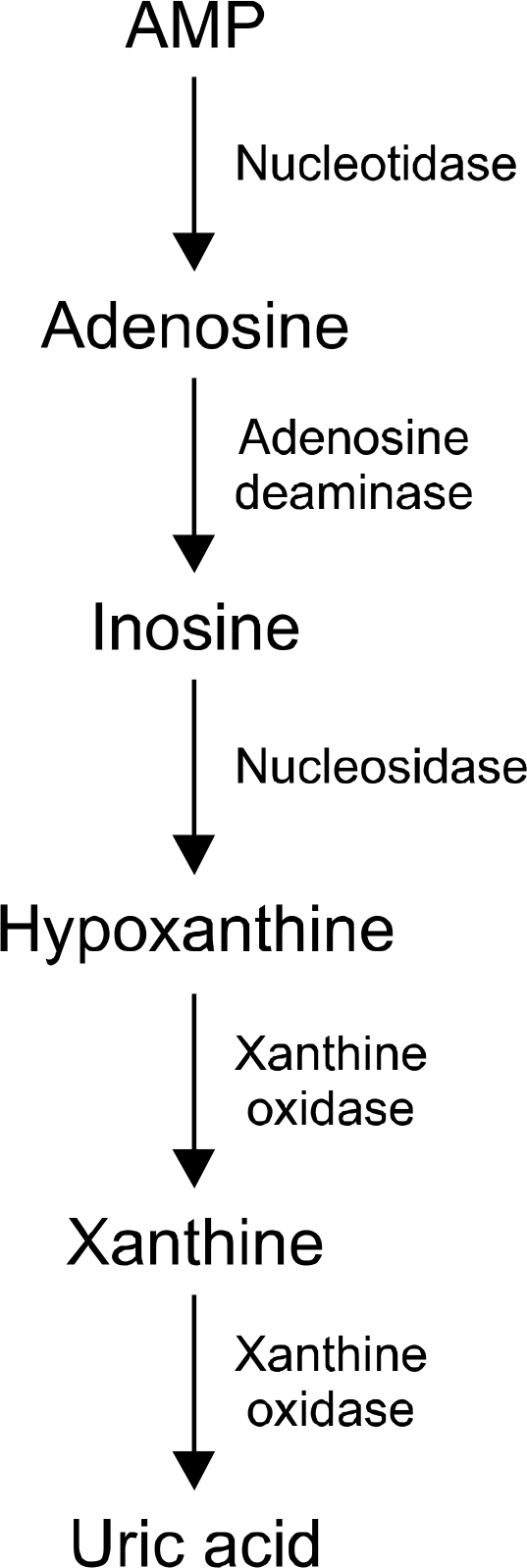
27. Voet D. Fundamentals of biochemistry. Life at the molecular level. 5th ed.Hoboken: John Wiley & Sons;2016. p. 1006–7.
28. Hong YS, Lee MJ, Kim KH, Lee SH, Lee YH, Kim BG, et al. The C677 mutation in methylene tetrahydrofolate reductase gene: correlation with uric acid and cardiovascular risk factors in elderly Korean men. J Korean Med Sci. 2004; 19:209–13.

29. Yao H, Ding LL, Wang XM, Xu FL. Polymophisms in methylenetetrahydrofolate reductase C677T and hyperuricemia in males. Ai Bian Ji Bian Tu Bian. 2007; 19:50–2.
30. Zhang QH, Liu J. Methylenetetrahydrofolate reductase C677T polymorphism for primary hyperuricemia and gout arthritis. Chin J Gen Pract. 2008; 7:259–60.
31. Wei W, Liu SY, Zeng FF, Ma L, Li KS, Wang BY. Meta-analysis of the association of the C677T polymorphism of the methylenetetrahydrofolate reductase gene with hyperuricemia. Ann Nutr Metab. 2012; 60:44–51.

32. Hinohara Y, Naito M, Okada R, Yin G, Higashibata T, Tamura T, et al. No association between MTHFR C677T and serum uric acid levels among Japanese with ABCG2 126QQ and SLC22A12 258WW. Nagoya J Med Sci. 2013; 75:93–100.
33. Pan YY. Association of the methylenetetrahydrofolate reductase polymorphism with hyperuricemia [thesis]. Yinchuan: Ningxia Medical University;. 2014.
34. Whitfield JB, Martin NG. Plasma lipids in twins. Environmental and genetic influences. Atherosclerosis. 1983; 48:265–77.
35. Emmerson BT, Nagel SL, Duffy DL, Martin NG. Genetic control of the renal clearance of urate: a study of twins. Ann Rheum Dis. 1992; 51:375–7.

36. Tuomilehto J, Zimmet P, Wolf E, Taylor R, Ram P, King H. Plasma uric acid level and its association with diabetes mellitus and some biologic parameters in a biracial population of Fiji. Am J Epidemiol. 1988; 127:321–36.

37. Motulsky AG. Nutritional ecogenetics: homocysteine-related arteriosclerotic vascular disease, neural tube defects, and folic acid. Am J Hum Genet. 1996; 58:17–20.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download