Abstract
A 36-yr-old woman with systemic lupus erythematosus was admitted in our center because of thrombocytopenia that was being treated with corticosteroids. She was prescribed a four-day course of intravenous immunoglobulin (IVIG) infusion. After three days of IVIG infusion, she developed aseptic meningitis with severe pleocytosis in the cerebrospinal fluid, followed by acute kidney injury. These complications resolved completely with conservative management. Here, we report these rare complications of IVIG and the outcome.
Immune thrombocytopenia purpura (ITP) is an acquired immune-mediated disorder characterized by isolated thrombocytopenia. ITP is observed relatively often in cases of systemic lupus erythematosus (SLE), wherein it is usually mild to moderate. However, in severe cases of ITP in such patients, when the platelet count is lower than 20,000/mm3 or when the thrombocytopenia is symptomatic, treatment with high-dose glucocorticoids or intravenous immunoglobulin (IVIG) should be considered [1]. It has been reported that IVIG acts via inhibition of Fc-receptor-mediated platelet phagocytosis and elimination of anti-platelet antibody production [2]. IVIG is generally efficacious and safe; however, its adverse effects, though rare, may be serious, even life threatening. Here, we report a case of aseptic meningitis and acute kidney injury after IVIG infusion in a 36-year-old woman with SLE-ITP.
Nine years prior, a 27-year-old woman was transferred to our hospital with easy bruising and petechiae due to severe thrombocytopenia (platelet count, 3,000/mm3). She was diagnosed with SLE according to the American College of Rheumatology classification (1997) based on the presence of antinuclear antibody titers of 1:640, oral ulcers, thrombocytopenia, and malar rash. At that time, laboratory tests showd white blood cell (WBC) count of 6,000/mm3 (lymphocytes, 32.8%), hemoglobin level of 11.6 g/dL (normal, 12~16 g/dL), hematocrit of 36.0% (normal, 36%~48%), creatinine level of 0.8 mg/dL (normal, 0.7~1.4 mg/dL), blood urea nitrogen level of 9 mg/dL (normal, 10~26 mg/dL), serum anti-double stranded DNA (dsDNA) antibody of 3.9 IU/mL (normal, <5 IU/mL), and Anti-Sm antibody was negative. Urine analysis showed no abnormal findings. Bone marrow examination was performed, and normocellular bone marrow with adequate megakaryopoiesis, which is consistent with ITP, was found. Anti-platelet antibody was found in her blood. Because of her severe SLE-ITP, she was initially treated with a high-dose glucocorticoid (methylprednisolone, 1 mg/kg). Consequently, the platelet count recovered to 67,000/mm3, and she was discharged with equivalent oral methylprednisolone and hydroxychloroquine (400 mg/day). After the platelet count recovered to 180,000/mm3, glucocorticoid treatment was tapered and then stopped after a total of 14 months.
However, her ITP had by then become glucocorticoid dependent, and platelet count had been unstable. One weeks prior, her platelet count decreased to 30,000/mm3 while maintaining methylprednisolone administration (4 mg/day). She was admitted for IVIG administration instead of raising the dose of glucocorticoids again.
At that time, her serum creatinine level was 0.68 mg/dL. There were no abnormal findings on urine analysis. On physical examination, numerous ecchymoses were observed on her limbs. Laboratory tests showed a WBC count of 3,900/mm3, hemoglobin level of 11.1 g/dL, platelet count of 30,000/mm3, erythrocyte sedimentation rate (ESR) of 10 mm/h (normal, <20 mm/h), and a serum C-reactive protein (CRP) level of 0.10 mg/dL (normal, <0.6 mg/dL). Her anti-dsDNA antibody levels was 3.9 IU/mL and C3 level was 101.0 mg/dL (normal, 90~180 mg/dL) and C4 level was 19.2 mg/dL (normal, 10~40 mg/dL).
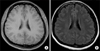
Maltose-containing IVIG (500 mg/kg/day) infusion was planned for four consecutive days. After three days of IVIG infusion, she developed fever and severe headache with nausea and vomiting. Examination of her cerebrospinal fluid (CSF) sample demonstrated a high opening pressure (27 cmH2O) and a WBC count of 1,350/mm3 with 89% segmented neutrophils. The CSF glucose level was within normal limits (61 mg/dL; range, 50~80 mg/dL), and CSF protein level was 131.9 mg/dL. Brain magnetic resonance imaging findings were normal (Figure 1). Empirical antibiotics (ceftriaxone and vancomycin) and dexamethasone (40 mg/day) were administered under suspicion of bacterial meningitis, and IVIG infusion was kept on hold. Two days after the last IVIG infusion, her headache and fever improved dramatically. CSF bacterial cultures and polymerase chain reaction viral test revealed negative results, and pleocytosis decreased to 110/mm3 in the follow-up CSF analysis. The aseptic meningitis was suggested as an adverse reaction to the IVIG treatment, and all antibiotics were discontinued.
At seven days after the last IVIG infusion, she developed oliguria and fever. CSF examination was again undertaken. Pleocytosis was not found (2/mm3), and the opening pressure was normal. However, in routine blood test, elevated blood urea nitrogen level (30 mg/dL), serum creatinine level (2.33 mg/dL), and acute phase reactants (ESR of 72 mm/h and CRP of 2.20 mg/dL) were detected. Sodium excretion fraction was identified to be 1.4%, and hydronephrosis or kidney stone was not found on non-contrast-enhanced abdominal computed tomography, suggesting acute kidney injury related to IVIG. After five days of 20 mg/day prednisolone treatment and hydration, serum creatinine level as well as her symptoms returned to normal. She was discharged with prednisolone 20 mg/day and hydroxychloroquine 400 mg/day. Headache and fever have not happened and renal function has not deteriorated again during the 12-month follow-up in the outpatient clinic, despite tapering the prednisolone dose to 5 mg/day. However, the platelet count has gradually decreased 9 months after the IVIG infusion (Figure 2).
Glucocorticoids are the first modality of treatment in SLE-ITP, and about 20% of the patients experience long-term remission. In cases of glucocorticoid-dependent or refractory ITP, the use of IVIG, danazol, cyclophosphamide, and rituximab has been suggested. Splenectomy is also often performed, though its effects are controversial [1].
Immunoglobulin replacement treatment was introduced in the 1950s for the treatment of primary immunodeficiency diseases. Treatment with IVIG greatly increased in the 1980s with the discovery of its efficacy in autoimmune and inflammatory diseases such as ITP. Currently, IVIG is used in the treatment of a wide variety of diseases, with more than 75% of the IVIG in the United States administered to patients with autoimmune or inflammatory conditions [3].
Adverse reactions to IVIG can be local (at the infusion site) or systemic. Systemic adverse reactions may involve the whole body or a specific organ (such as kidney or skin) or system (such as the nervous or hematologic system). The most common immediate adverse reactions are chills, fever, headache, and muscular pain [45]. The rates of adverse events vary considerably depending on the type of patient and the adjuvant of the immunoglobulin used [5]. Rare adverse reactions such as anaphylaxis, aseptic meningitis, acute kidney injury, thromboembolism, and hemolysis were also reported [5].
Aseptic meningitis after IVIG was first reported in 1988. Since then, there have been similar reports of IVIG-induced meningitis in patients with medical conditions such as idiopathic thrombocytopenic purpura, myasthenia gravis, and inflammatory demyelinating neuropathy [56]. The rate of aseptic meningitis after IVIG varies widely, from 0% to 11%, in patients with different underlying diseases [7]. The cause of aseptic meningitis is not known but its occurrence with high-dose IVIG administration suggests that the central nervous system inflammatory response may result from small quantities of immunoglobulin G entering the CSF and causing inflammation and osmotic shifts of the meninges [178]. The CSF analysis in these cases commonly showed mild to moderate pleocytosis and a high protein level, which resolved almost completely within 10 days [6]. Although the pleocytosis in our case was more than 1,000/mm3, which is unusual in IVIG-induced aseptic meningitis, the patient recovered completely within four days of cessation of IVIG administration without maintaining antibiotic treatment.
Acute kidney injury following IVIG infusion is a very rare complication. The recovery of renal function usually occurs within ten days [9]. IVIG-related acute kidney injury has been known to occur mainly with saccharose-containing preparations of IVIG, but rarely it may occur with maltose- and glucose-containing preparations of IVIG [9]. Prevention and treatment includes evaluating renal function prior to treatment, prehydration, avoiding volume depletion by diuretics, using slow infusion rates, and limiting the IVIG dose to no more than 0.5 g/kg/day [59101112].
We report a rare case of aseptic meningitis following acute kidney injury, which are serious complications of IVIG infusion in patients with SLE-ITP. These complications resolved completely by stopping IVIG and supportive care. We should be aware of the serious complications related with IVIG and appropriate actions are required promptly when these complications are suspected.
Figures and Tables
References
1. Newman K, Owlia MB, El-Hemaidi I, Akhtari M. Management of immune cytopenias in patients with systemic lupus erythematosus - Old and new. Autoimmun Rev. 2013; 12:784–791.
2. Hansen RJ, Balthasar JP. Mechanisms of IVIG action in immune thrombocytopenic purpura. Clin Lab. 2004; 50:133–140.
3. Gelfand EW. Intravenous immune globulin in autoimmune and inflammatory diseases. N Engl J Med. 2012; 367:2015–2025.
4. Duhem C, Dicato MA, Ries F. Side-effects of intravenous immune globulins. Clin Exp Immunol. 1994; 97:Suppl 1. 79–83.
5. Stiehm ER. Adverse effects of human immunoglobulin therapy. Transfus Med Rev. 2013; 27:171–178.
6. Bharath V, Eckert K, Kang M, Chin-Yee IH, Hsia CC. Incidence and natural history of intravenous immunoglobulin-induced aseptic meningitis: a retrospective review at a single tertiary care center. Transfusion. 2015; 55:2597–2605.
7. Kemmotsu Y, Nakayama T, Matsuura H, Saji T. Clinical characteristics of aseptic meningitis induced by intravenous immunoglobulin in patients with Kawasaki disease. Pediatr Rheumatol Online J. 2011; 9:28.
8. Jain RS, Kumar S, Aggarwal R, Kookna JC. Acute aseptic meningitis due to intravenous immunoglobulin therapy in Guillain-Barré syndrome. Oxf Med Case Reports. 2014; 2014:132–134. DOI: 10.1093/omcr/omu051. eCollection 2014.
9. Fakhouri F. Intravenous immunoglobulins and acute renal failure: mechanism and prevention. Rev Med Interne. 2007; 28(Spec No. 1):4–6.
10. Ahsan N. Intravenous immunoglobulin induced-nephropathy: a complication of IVIG therapy. J Nephrol. 1998; 11:157–161.
11. Angeli P, Scaglione F. Nephrotoxicity of intravenous immunoglobulin in the setting of liver transplantation or HBV-related cirrhosis: an undervalued topic. Minerva Gastroenterol Dietol. 2008; 54:259–275.
12. Levy JB, Pusey CD. Nephrotoxicity of intravenous immunoglobulin. QJM. 2000; 93:751–755.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download