Abstract
Tocilizumab, a humanized monoclonal antibody against the interleukin-6 receptor, is therapeutically effective in patients diagnosed with rheumatoid arthritis (RA) compared with placebo. However patients treated with tocilizumab are at increased risk of several adverse effects including anaphylaxis and serious infections that may lead to hospitalization or death. Therefore, the risks and benefits of treatment with tocilizumab should be considered carefully and close monitoring of patients for development of signs and symptoms of side effects is required during and after treatment. Here, we report on a rare case of anaphylaxis and severe sepsis caused by cellulitis in a patient with RA after tocilizumab treatment.
REFERENCES
1. Keyser FD. Choice of biologic therapy for patients with rheumatoid arthritis: the infection perspective. Curr Rheumatol Rev. 2011; 7:77–87.
2. Ellerin T, Rubin RH, Weinblatt ME. Infections and anti-tumor necrosis factor alpha therapy. Arthritis Rheum. 2003; 48:3013–22.
3. Strangfeld A, Listing J. Infection and musculoskeletal conditions: bacterial and opportunistic infections during an-ti-TNF therapy. Best Pract Res Clin Rheumatol. 2006; 20:1181–95.
5. Jones G, Sebba A, Gu J, Lowenstein MB, Calvo A, Gomez-Reino JJ, et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis. 2010; 69:88–96.

6. Smolen JS, Beaulieu A, Rubbert-Roth A, Ramos-Remus C, Rovensky J, Alecock E, et al. OPTION Investigators. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a dou-ble-blind, placebo-controlled, randomised trial. Lancet. 2008; 371:987–97.

7. Hashimoto J, Garnero P, van der Heijde D, Miyasaka N, Yamamoto K, Kawai S, et al. Humanized anti-interleukin-6-receptor antibody (tocilizumab) monotherapy is more effective in slowing radiographic progression in patients with rheumatoid arthritis at high baseline risk for structural damage evaluated with levels of biomarkers, radiography, and BMI: data from the SAMURAI study. Mod Rheumatol. 2011; 21:10–5.

8. Genentech, Inc. The manual of Actemra (tocilizumab). San Francisco, CA, Genentech: Inc.;2013.
9. Galvão VR, Castells MC. Hypersensitivity to biological agents-updated diagnosis, management, and treatment. J Allergy Clin Immunol Pract. 2015; 3:175–85.

10. Naugler WE, Karin M. The wolf in sheep's clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol Med. 2008; 14:109–19.

12. Hirao M, Nampei A, Shi K, Yoshikawa H, Nishimoto N, Hashimoto J. Diagnostic features of mild cellulitis phlegmon in patients with rheumatoid arthritis treated with tocilizumab: a report of two cases. Mod Rheumatol. 2011; 21:673–7.

13. The Korean Society of Infectious Diseases; The Korean Society for Chemotherapy; The Korean Orthopaedic Asso-ciation; The Korean Society of Clinical Microbiology. Korean Dermatological Association. Clinical practice guidelines for soft tissue infections. Infect Chemother. 2012; 44:213–32.
14. Chuang HC, Ho YH, Lay CJ, Wang LS, Tsai YS, Tsai CC. Different clinical characteristics among aeromonas hydro-phila, aeromonas veronii biovar sobria and aeromonas cav-iae monomicrobial bacteremia. J Korean Med Sci. 2011; 26:1415–20.
Figure 1.
Four days after the second intravenous administration of tocilizumab, edema and ecchymosis developed in the both lower limbs (A) and hemorrhagic blisters developed on the left dorsal foot (B).
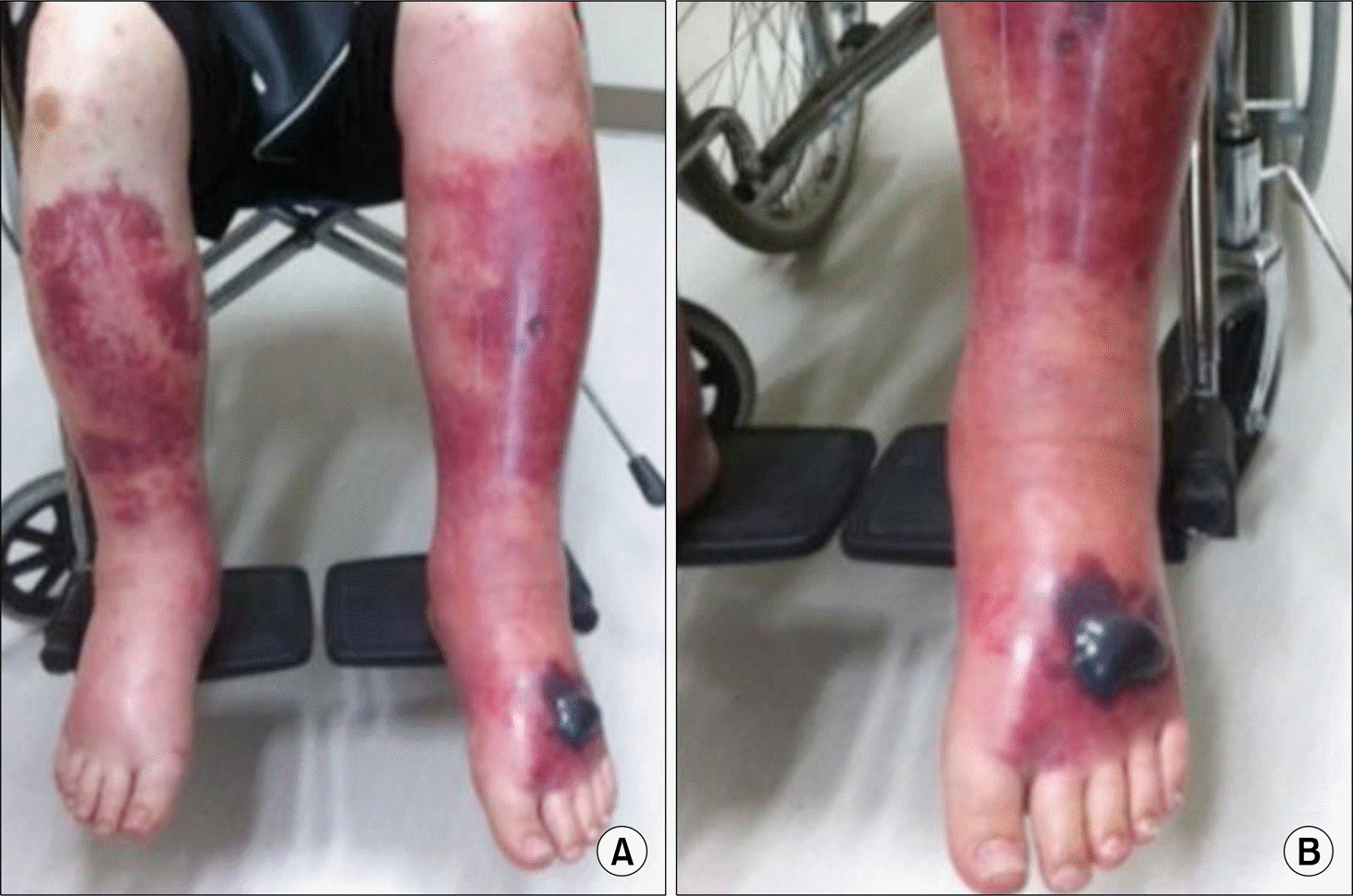
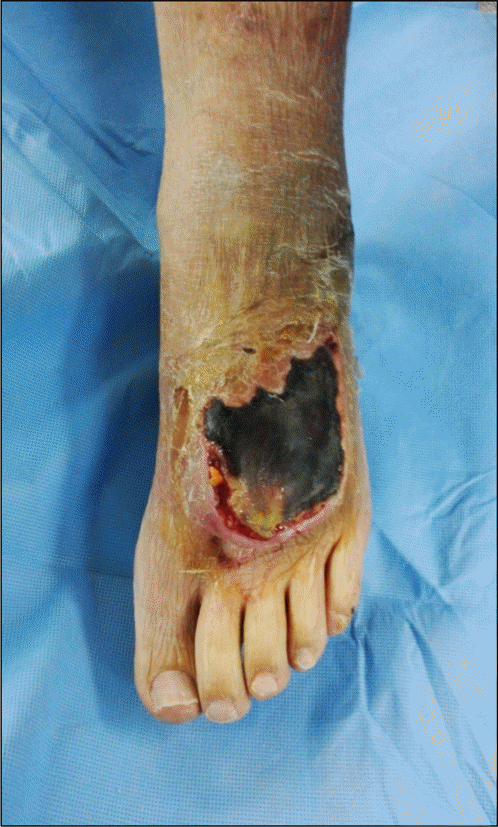
Figure 2.
(A) Axial T2-weighted magnetic resonance (MR), left lower leg. (B) Axial T1-weighted MR, left lower leg. (C) Axial T1-weighted post contrast MR, left lower leg. (D) Axial T2-weighted MR, left lower leg. (E) Axial T1-weighted MR, right lower leg.(F) Axial T1-weighted post contrast MR, right lower leg. Subcutaneous thickening with fluid collections (arrows) was revealed on T2-weighted images and subcutaneous tissue and superficial fascia (arrowheads) showed contrast enhancement.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download