Abstract
Objective
The aim of this study was to examine and compare the gastrointestinal (GI) risk factors and treatment patterns of rheumatoid arthritis (RA) and osteoarthritis (OA) patients in Korea.
Methods
This was a cross-sectional, observational study on RA and OA patients taking non-steroidal anti-inflammatory drugs (NSAIDs) for at least 1 month. A total of 1,896 patients (981 RA patients, 915 OA patients) were recruited from 20 university hospitals. Data were collected through medical records and patient surveys. GI risk factors included age, prolonged (over 3 months) or high-dose use of NSAIDs, alcohol drinking, smoking, use of aspirin, anticoagulants or glucocorticoids, comorbidities, and history of Helicobacter pylori infection or other GI complications. Treatment patterns were classified according to groups using, selective cyclooxygenase (COX)-2 in-hibitors±gastroprotective agents, non-selective COX-2 inhibitors+proton pump inhibitor, or non-selective COX-2 in-hibitors±other gastroprotective agents.
Results
GI risk factors were highly present in both RA and OA patients. The proportion of prolonged use of NSAIDs, smoking, and glucocorticoid use were higher in RA patients (p<0.001). The proportion of comorbidities and use of aspirin were higher in OA patients (p<0.001). The remaining GI risk factors were present in similar proportions in both groups. Use of selective COX-2 inhibitors or gastroprotective agents was higher in RA patients.
Conclusion
Prolonged use of NSAIDs and concomitant glucocorticoid use were higher in RA patients, while comorbidities and concomitant aspirin use were predominant in OA patients. These results will provide insights for use in development of future guidelines for proper selection of NSAIDs and effective prevention of GI complications in arthritis patients.
Go to : 
REFERENCES
2. Rahme E, Joseph L, Kong SX, Watson DJ, LeLorier J. Gastrointestinal health care resource use and costs associated with nonsteroidal antiinflammatory drugs versus acetaminophen: retrospective cohort study of an elderly population. Arthritis Rheum. 2000; 43:917–24.

3. Smalley WE, Griffin MR, Fought RL, Ray WA. Excess costs from gastrointestinal disease associated with nonsteroidal anti-inflammatory drugs. J Gen Intern Med. 1996; 11:461–9.

4. Bloom BS. Direct medical costs of disease and gastrointestinal side effects during treatment for arthritis. Am J Med. 1988; 84(2A):20–4.

5. American Gastroenterological Association. Wilcox CM, Allison J, Benzuly K, Borum M, Cryer B, et al. Consensus development conference on the use of nonsteroidal anti-inflammatory agents, including cyclooxygenase-2 enzyme inhibitors and aspirin. Clin Gastroenterol Hepatol. 2006; 4:1082–9.

6. Rostom A, Moayyedi P, Hunt R. Canadian Association of Gastroenterology Consensus Group. Canadian consensus guidelines on long-term nonsteroidal anti-inflammatory drug therapy and the need for gastroprotection: benefits versus risks. Aliment Pharmacol Ther. 2009; 29:481–96.

7. Lanza FL, Chan FK, Quigley EM. Practice Parameters Committee of the American College of Gastroenterology. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009; 104:728–38.

8. Abraham NS, El-Serag HB, Johnson ML, Hartman C, Richardson P, Ray WA, et al. National adherence to evidence-based guidelines for the prescription of nonsteroidal anti-inflammatory drugs. Gastroenterology. 2005; 129:1171–8.

9. Laine L, Connors L, Griffin MR, Curtis SP, Kaur A, Cannon CP. Prescription rates of protective co-therapy for NSAID users at high GI risk and results of attempts to improve adherence to guidelines. Aliment Pharmacol Ther. 2009; 30:767–74.

10. Singh G, Triadafilopoulos G. Epidemiology of NSAID induced gastrointestinal complications. J Rheumatol Suppl. 1999; 56:18–24.
11. Fries JF. NSAID GI toxicity: epidemiology. J Musculoskel Med. 1991; 8:21–8.
12. Fries JF, Williams CA, Bloch DA, Michel BA. Nonsteroidal anti-inflammatory drug-associated gastropathy: incidence and risk factor models. Am J Med. 1991; 91:213–22.

13. Piper JM, Ray WA, Daugherty JR, Griffin MR. Corticosteroid use and peptic ulcer disease: role of nonsteroidal antiinflammatory drugs. Ann Intern Med. 1991; 114:735–40.

14. Shorr RI, Ray WA, Daugherty JR, Griffin MR. Concurrent use of nonsteroidal anti-inflammatory drugs and oral anti-coagulants places elderly persons at high risk for hemorrhagic peptic ulcer disease. Arch Intern Med. 1993; 153:1665–70.

15. Gabriel SE, Jaakkimainen L, Bombardier C. Risk for serious gastrointestinal complications related to use of non-steroidal anti-inflammatory drugs. A meta-analysis. Ann Intern Med. 1991; 115:787–96.
16. Singh G, Rosen Ramey D. NSAID induced gastrointestinal complications: the ARAMIS perspective-1997. Arthritis, Rheumatism, and Aging Medical Information System. J Rheumatol Suppl. 1998; 51:8–16.
17. Gutthann SP, García Rodríguez LA, Raiford DS. Individual nonsteroidal antiinflammatory drugs and other risk factors for upper gastrointestinal bleeding and perforation. Epidemiology. 1997; 8:18–24.

18. Silverstein FE, Graham DY, Senior JR, Davies HW, Struthers BJ, Bittman RM, et al. Misoprostol reduces serious gastrointestinal complications in patients with rheumatoid arthritis receiving nonsteroidal anti-inflammatory drugs. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1995; 123:241–9.
19. McIntosh JH, Fung CS, Berry G, Piper DW. Smoking, non-steroidal anti-inflammatory drugs, and acetaminophen in gastric ulcer. A study of associations and of the effects of previous diagnosis on exposure patterns. Am J Epidemiol. 1988; 128:761–70.
20. Singh G. Recent considerations in nonsteroidal anti-inflammatory drug gastropathy. Am J Med. 1998; 105:31S–8S.

21. Lee SH, Han CD, Yang IH, Ha CW. Prescription pattern of NSAIDs and the prevalence of NSAID-induced gastrointestinal risk factors of orthopaedic patients in clinical practice in Korea. J Korean Med Sci. 2011; 26:561–7.

22. Lanas A, Garcia-Tell G, Armada B, Oteo-Alvaro A. Prescription patterns and appropriateness of NSAID therapy according to gastrointestinal risk and cardiovascular history in patients with diagnoses of osteoarthritis. BMC Med. 2011; 9:38.

23. Laine L, Curtis SP, Cryer B, Kaur A, Cannon CP. Risk factors for NSAID-associated upper GI clinical events in a long-term prospective study of 34 701 arthritis patients. Aliment Pharmacol Ther. 2010; 32:1240–8.
Go to : 
 | Figure 1.Gastrointestinal (GI) risk factors in rheumatoid arthritis and osteoarthritis patients (A: all age, B: <60 yr, C: ≥60 yr). NSAID: non-steroidal anti-inflammatory drug. *p<0.001 by chi-square test. |
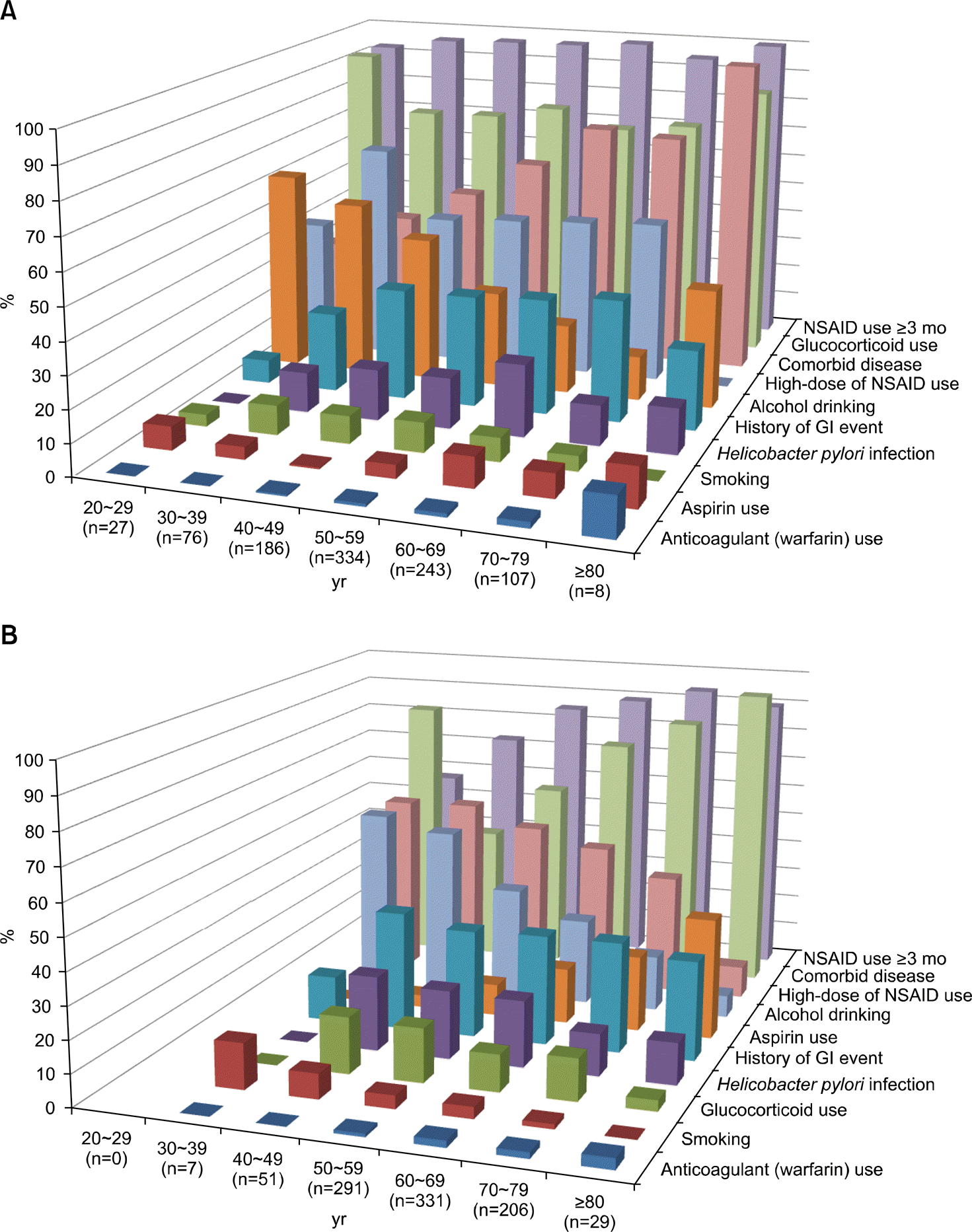 | Figure 2.Gastrointestinal (GI) risk factors stratified by 10 years age in rheumatoid arthritis and osteoarthritis patients (A: Rheu-matoid arthritis, B: Osteoarthritis). NSAID: non-steroidal anti-inflammatory drug. |
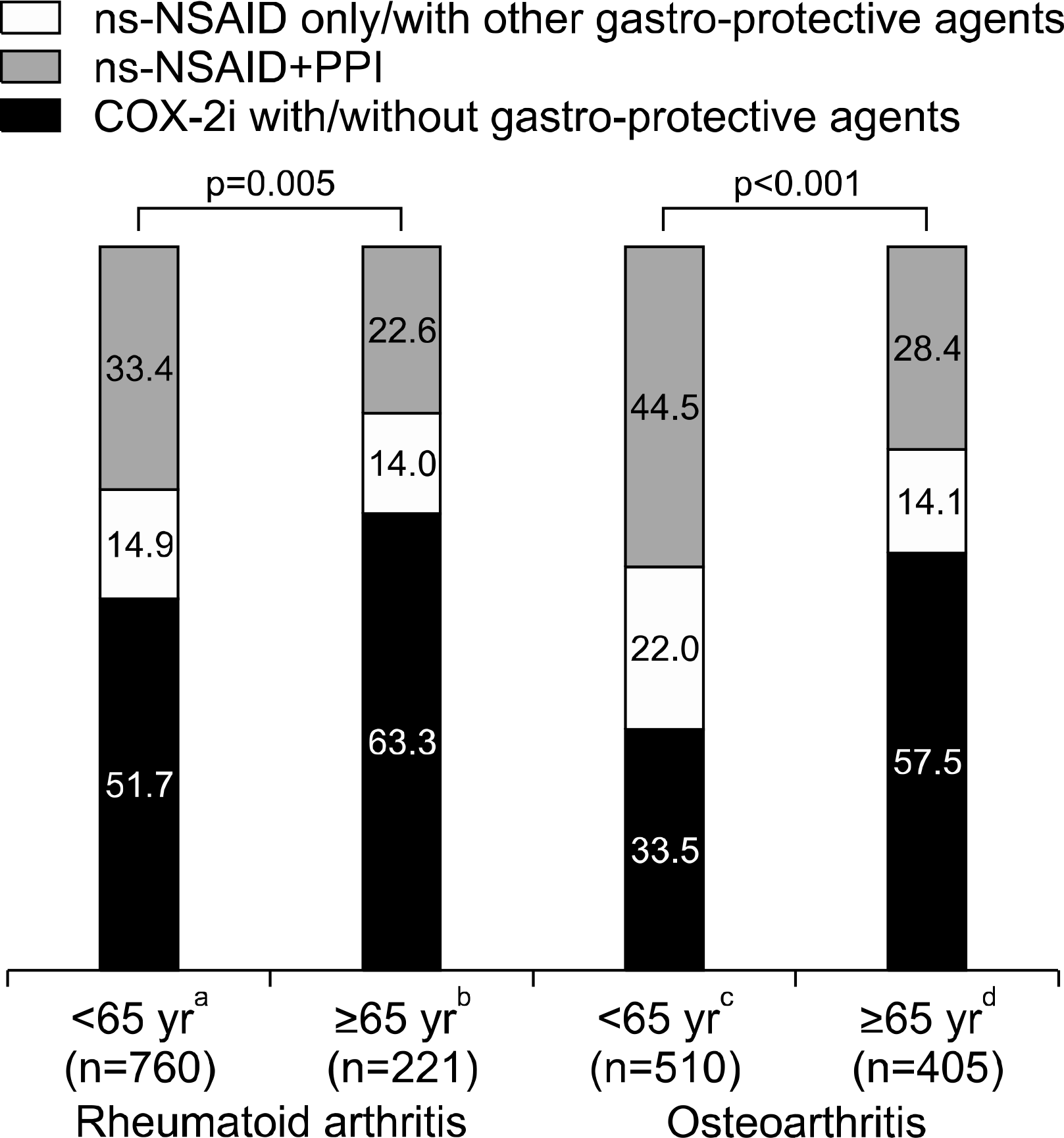 | Figure 3.Treatment patterns of NSAIDs by age. Values are presented as percent only. COX-2i: selective cycloxygenase-2 inhibitor, ns-NSAID: non-selective non-steroidal antiinflammatory drug, PPI: proton pump inhibitor. a vs. c, p<0.001; b vs. d, p=0.270. |
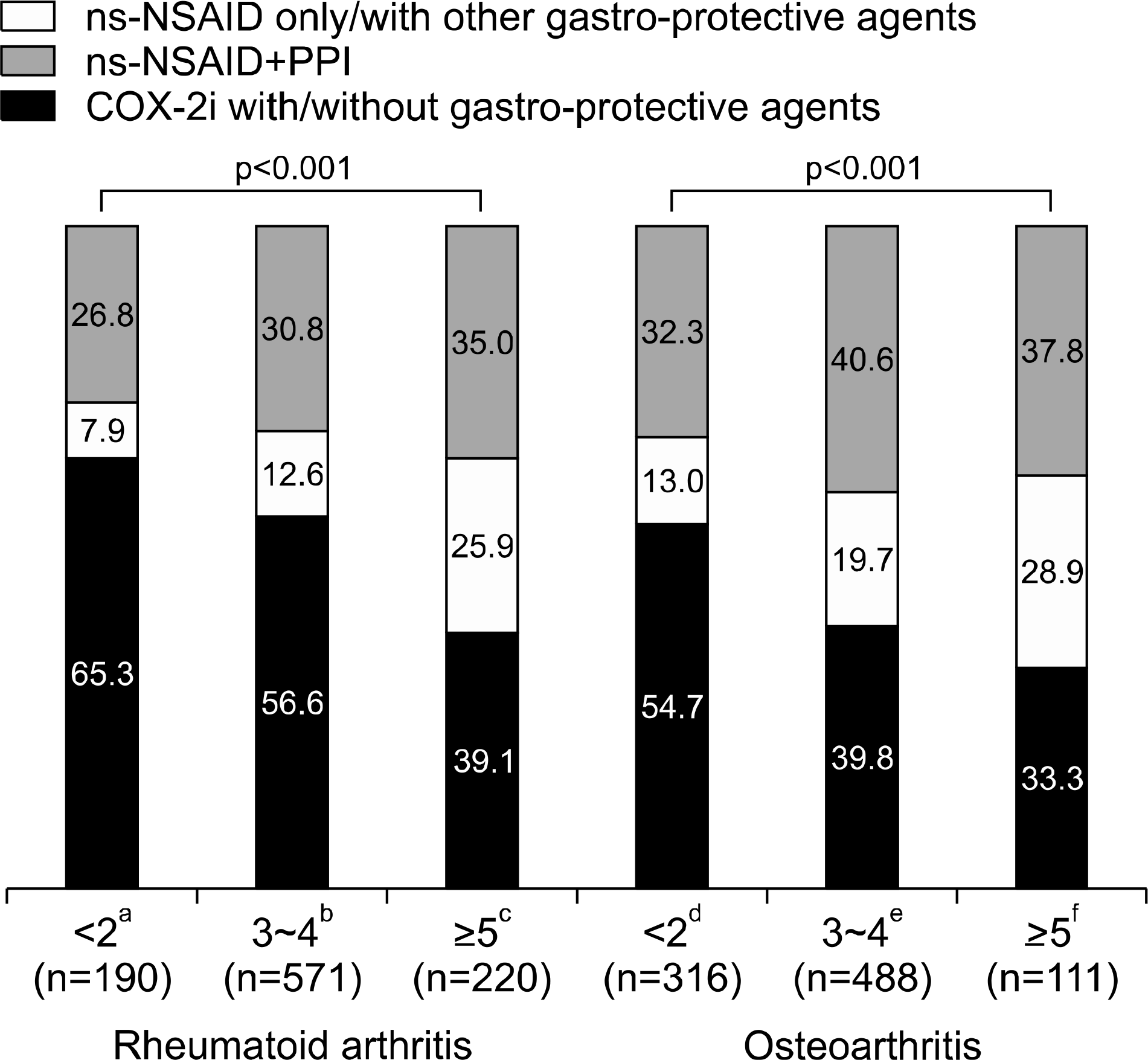 | Figure 4.Treatment patterns in terms of gastrointestinal risk factor numbers. Values are presented as percent only. COX-2i: selective cycloxygenase-2 inhibitor, ns-NSAID: non-selective non-steroidal anti-inflammatory drug, PPI: proton pump inhibitor. a vs. d, p=0.046; b vs. e, p<0.001; c vs. f, p=0.589. |
Table 1.
Characteristics of rheumatoid arthritis and osteoarthritis patients
| Variable | Rheumatoid arthritis (n=981) | Osteoarthritis (n=915) |
|---|---|---|
| Age (yr)* | 55.0±11.7 | 62.9±9.2 |
| <60 | 623 (63.5) | 349 (38.1) |
| ≥60 | 358 (36.5) | 566 (61.9) |
| <65 | 760 (77.5) | 510 (55.7) |
| ≥65 | 221 (22.5) | 405 (44.3) |
| Sex* | ||
| Male | 146 (14.9) | 82 (9.0) |
| Female | 835 (85.1) | 833 (91.0) |
| Disease duration (mo)* | 73.0±68.8 | 49.9±50.7 |
| Median (IQR) | 55 (22∼104) | 33 (11∼72) |
| Range | 1∼548 | 1∼407 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download