Abstract
Objective
The purpose of this study is to evaluate the clinical and hematological effects of tocilizumab in active rheumatoid arthritis (RA) patients.
Methods
Fourteen patients with active RA were enrolled in this study. The patients received tocilizumab 8 mg/kg intravenously every four weeks for 6 months. Disease activity, anemia-related factors including serum hepcidin-25, and hematological parameters were monitored at baseline and at 1, 3, and 6 months after the initiation of treatment.
Results
Significant reductions in tender joint count, swollen joint count, visual analogue scale, erythrocyte sedimentation rate (ESR), and C-reactive (CRP) protein plus reductions in a 28-joint disease activity score were observed within one month after the first tocilizumab treatment. These effects lasted throughout the six-month study period. In addition, significant improvements in anemia-related factors such as hepcidin-25, ferritin, iron, hemoglobin, red blood cell counts and mean corpuscular volume were observed during the treatment period. Hematological parameters were improved with reductions in counts for leukocytes, monocytes, neutrophils, and platelets. The lymphocyte counts and their subset numbers were unchanged. Changes in hepcidin levels showed significant correlation with changes in CRP, ESR, ferritin, hemoglobin and counts for red blood cells, leukocytes, and neutrophils during the treatment period.
Conclusion
This study demonstrates that tocilizumab significantly and meaningfully reduces disease burden in patients with active RA. In addition, tocilizumab diminishes the levels of inflammatory anemia by inhibiting hepcidin production. These clinical data provide evidence of a favorable outcome from tocilizumab in RA.
Go to : 
REFERENCES
1. McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007; 7:429–42.

3. Hashizume M, Mihara M. The roles of interleukin-6 in the pathogenesis of rheumatoid arthritis. Arthritis. 2011; 2011; 765624.
4. Wilson A, Yu HT, Goodnough LT, Nissenson AR. Prevalence and outcomes of anemia in rheumatoid arthritis: a systematic review of the literature. Am J Med. 2004; 116(Suppl 7A):50S–7S.

6. Voulgari PV, Kolios G, Papadopoulos GK, Katsaraki A, Seferiadis K, Drosos AA. Role of cytokines in the pathogenesis of anemia of chronic disease in rheumatoid arthritis. Clin Immunol. 1999; 92:153–60.

7. Romano M, Sironi M, Toniatti C, Polentarutti N, Fruscella P, Ghezzi P, et al. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity. 1997; 6:315–25.

8. Hashizume M, Hayakawa N, Mihara M. IL-6 trans-signal-ling directly induces RANKL on fibroblast-like synovial cells and is involved in RANKL induction by TNF-alpha and IL-17. Rheumatology. 2008; 47:1635–40.
9. Hashizume M, Hayakawa N, Suzuki M, Mihara M. IL-6/sIL-6R trans-signalling, but not TNF-alpha induced angiogenesis in a HUVEC and synovial cell co-culture system. Rheumatol Int. 2009; 29:1449–54.
10. Madhok R, Crilly A, Watson J, Capell HA. Serum interleukin 6 levels in rheumatoid arthritis: correlations with clinical and laboratory indices of disease activity. Ann Rheum Dis. 1993; 52:232–4.

11. Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004; 113:1271–6.

12. Roy C, Andrews NC. Anemia of inflammation: the hepcidin link. Curr Opin Hematol. 2005; 12:107–11.

13. Kawabata H, Tomosugi N, Kanda J, Tanaka Y, Yoshizaki K, Uchiyama T. Anti-interleukin 6 receptor antibody tocilizumab reduces the level of serum hepcidin in patients with multicentric Castleman's disease. Haematologica. 2007; 92:857–8.

14. Song S, Tomosugi N, Kawabata H, Ishikawa T, Nishikawa T, Yoshizaki K. Down-regulation of hepcidin resulting from long-term treatment with an anti-IL-6 receptor antibody (tocilizumab) improves anemia of inflammation in multicentric Castleman disease. Blood. 2010; 116:3627–34.

15. Hashizume M, Uchiyama Y, Horai N, Tomosugi N, Mihara M. Tocilizumab, a humanized anti-interleukin-6 receptor antibody, improved anemia in monkey arthritis by suppressing IL-6-induced hepcidin production. Rheumatol Int. 2010; 30:917–23.

16. Isaacs JD, Harari O, Kobold U, Lee JS, Bernasconi C. Effect of tocilizumab on haematological markers implicates interleukin-6 signalling in the anaemia of rheumatoid arthritis. Arthritis Res Ther. 2013; 15:R204.

17. Song SN, Iwahashi M, Tomosugi N, Uno K, Yamana J, Yamana S, et al. Comparative evaluation of the effects of treatment with tocilizumab and TNF-α inhibitors on serum hepcidin, anemia response and disease activity in rheumatoid arthritis patients. Arthritis Res Ther. 2013; 15:R141.

18. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/ European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010; 62:2569–81.
19. Park YW, Kee SJ, Cho YN, Lee EH, Lee HY, Kim EM, et al. Impaired differentiation and cytotoxicity of natural killer cells in systemic lupus erythematosus. Arthritis Rheum. 2009; 60:1753–63.

20. Cho YN, Kee SJ, Lee SJ, Seo SR, Kim TJ, Lee SS, et al. Numerical and functional deficiencies of natural killer T cells in systemic lupus erythematosus: their deficiency related to disease activity. Rheumatology (Oxford). 2011; 50:1054–63.

21. Cho YN, Kee SJ, Kim TJ, Jin HM, Kim MJ, Jung HJ, et al. Mucosal-associated invariant T cell deficiency in systemic lupus erythematosus. J Immunol. 2014; 193:3891–901.

22. Lee SJ, Cho YN, Kim TJ, Park SC, Park DJ, Jin HM, et al. Natural killer T cell deficiency in active adult-onset Still's disease: correlation of deficiency of natural killer T cells with dysfunction of natural killer cells. Arthritis Rheum. 2012; 64:2868–77.

23. Jin HM, Kee SJ, Cho YN, Kang JH, Kim MJ, Jung HJ, et al. Dysregulated osteoclastogenesis is related to natural killer T cell dysfunction in rheumatoid arthritis. Arthritis Rheumatol. 2015; 67:2639–50.

24. Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acutephase protein. Blood. 2003; 101:2461–3.

25. Yokota S, Miyamae T, Imagawa T, Iwata N, Katakura S, Mori M, et al. Therapeutic efficacy of humanized recombinant an-ti-interleukin-6 receptor antibody in children with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2005; 52:818–25.

26. van Santen S, van Dongen-Lases EC, de Vegt F, Laarakkers CM, van Riel PL, van Ede AE, et al. Hepcidin and hemoglobin content parameters in the diagnosis of iron deficiency in rheumatoid arthritis patients with anemia. Arthritis Rheum. 2011; 63:3672–80.

27. Koca SS, Isik A, Ustundag B, Metin K, Aksoy K. Serum pro-hepcidin levels in rheumatoid arthritis and systemic lupus erythematosus. Inflammation. 2008; 31:146–53.

28. Kim HR, Kim KW, Yoon SY, Kim SH, Lee SH. Serum pro-hepcidin could reflect disease activity in patients with rheumatoid arthritis. J Korean Med Sci. 2010; 25:348–52.

29. Sellam J, Kotti S, Fellahi S, Bastard JP, Meyer M, Lioté F, et al. Serum hepcidin level is not an independent surrogate biomarker of disease activity or of radiographic progression in rheumatoid arthritis: results from the ESPOIR cohort. Ann Rheum Dis. 2013; 72:312–4.

30. Maslak P, Nimer SD. The efficacy of IL-3, SCF, IL-6, and IL-11 in treating thrombocytopenia. Semin Hematol. 1998; 35:253–60.
31. Suwa T, Hogg JC, English D, Van Eeden SF. Interleukin-6 induces demargination of intravascular neutrophils and shortens their transit in marrow. Am J Physiol Heart Circ Physiol. 2000; 279:H2954–60.

32. Muraguchi A, Hirano T, Tang B, Matsuda T, Horii Y, Nakajima K, et al. The essential role of B cell stimulatory factor 2 (BSF-2/IL-6) for the terminal differentiation of B cells. J Exp Med. 1988; 167:332–44.

33. Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007; 8:967–74.

34. Illei GG, Shirota Y, Yarboro CH, Daruwalla J, Tackey E, Takada K, et al. Tocilizumab in systemic lupus erythematosus: data on safety, preliminary efficacy, and impact on circulating plasma cells from an open-label phase I dos-age-escalation study. Arthritis Rheum. 2010; 62:542–52.

35. Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002; 110:1037–44.

36. Nishimoto N, Terao K, Mima T, Nakahara H, Takagi N, Kakehi T. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood. 2008; 112:3959–64.

Go to : 
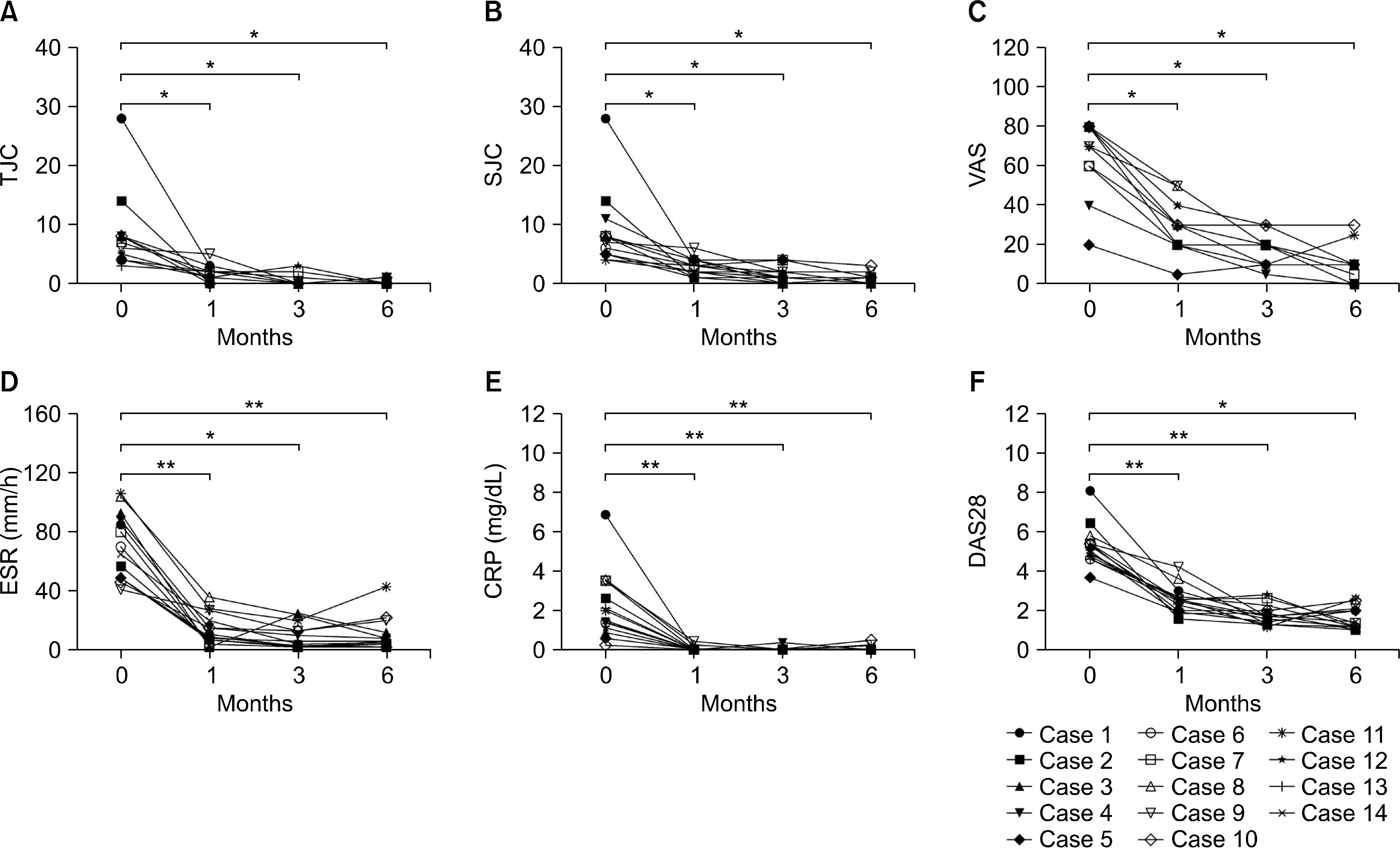 | Figure 1.Clinical effects of tocilizumab in rheumatoid arthritis (RA) patients during six-month treatment period. Tocilizumab was administrated to 14 RA patients every four weeks. Disease activity was assessed at baseline and months 1, 3 and 6 during tocilizumab treatment. (A) Tender joint count (TJC). (B) Swollen joint count (SJC). (C) Visual analogue scale (VAS). (D) Erythrocyte sedimentation rate (ESR). (E) C-reactive protein (CRP). (F) 28-joint disease activity score (DAS28). Symbols represent individual subjects. p-values were determined using Wilcoxon's signed rank test. *p<0.005, **p<0.0005. |
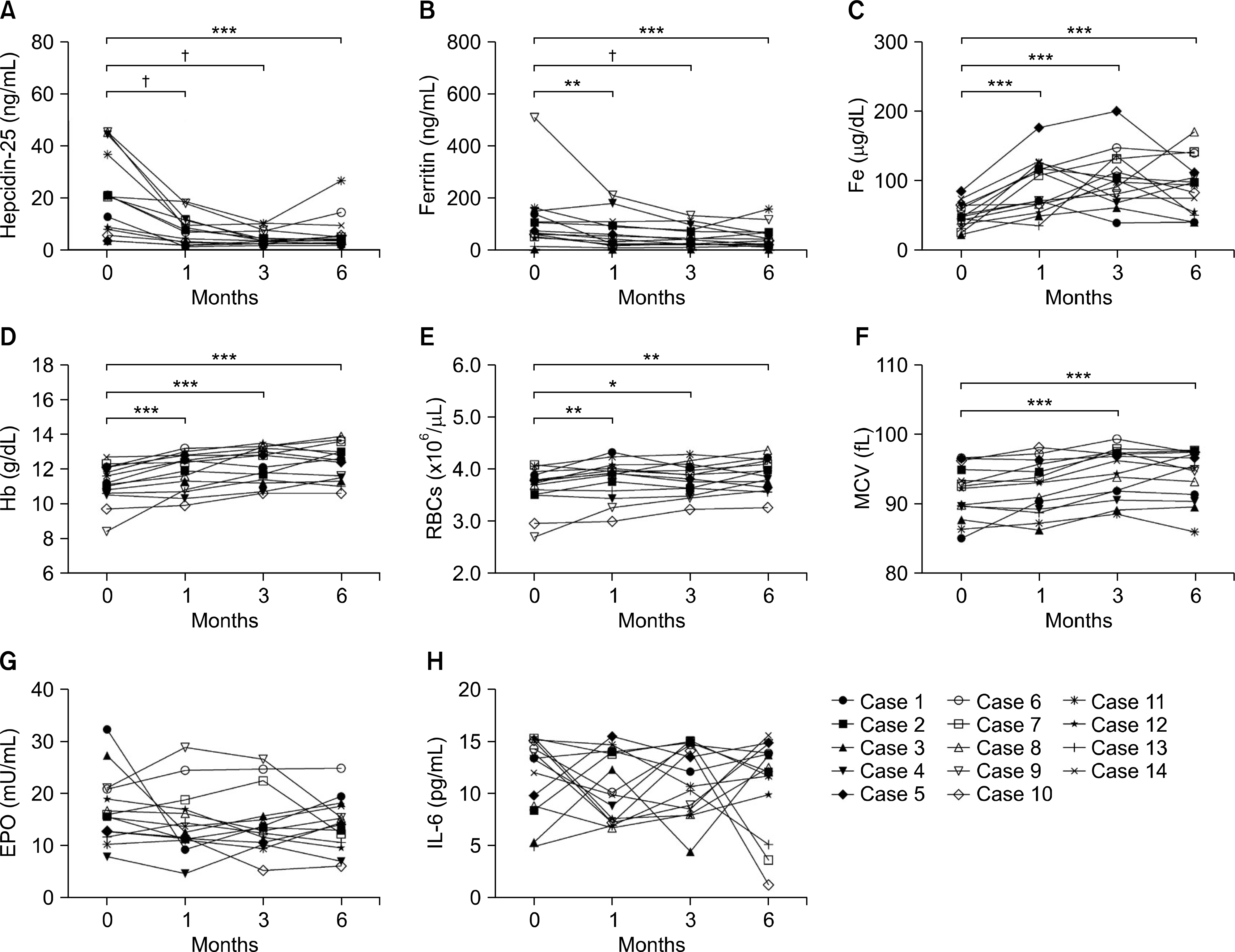 | Figure 2.Effects of tocilizumab on hepcidin-25 and anemia-related factors in rheumatoid arthritis (RA) patients during the six-month treatment period. Tocilizumab was administrated to 14 RA patients every four weeks. Hepcidin-25 and anemia-related factors were measured at baseline and months 1, 3 and 6 during tocilizumab treatment. (A) Serum hepcidin-25. (B) Serum ferritin.(C) Serum iron (Fe). (D) Hemoglobin (Hb). (E) Red blood cell counts (RBCs). (F) Mean corpuscular volume (MCV). (G) Erythropoietin (EPO). (H) Interleukin (IL)-6. Symbols represent individual subjects. p-values were determined using Wilcoxon's signed rank test.*p<0.05, **p<0.01, ***p<0.005, † p<0.001. |
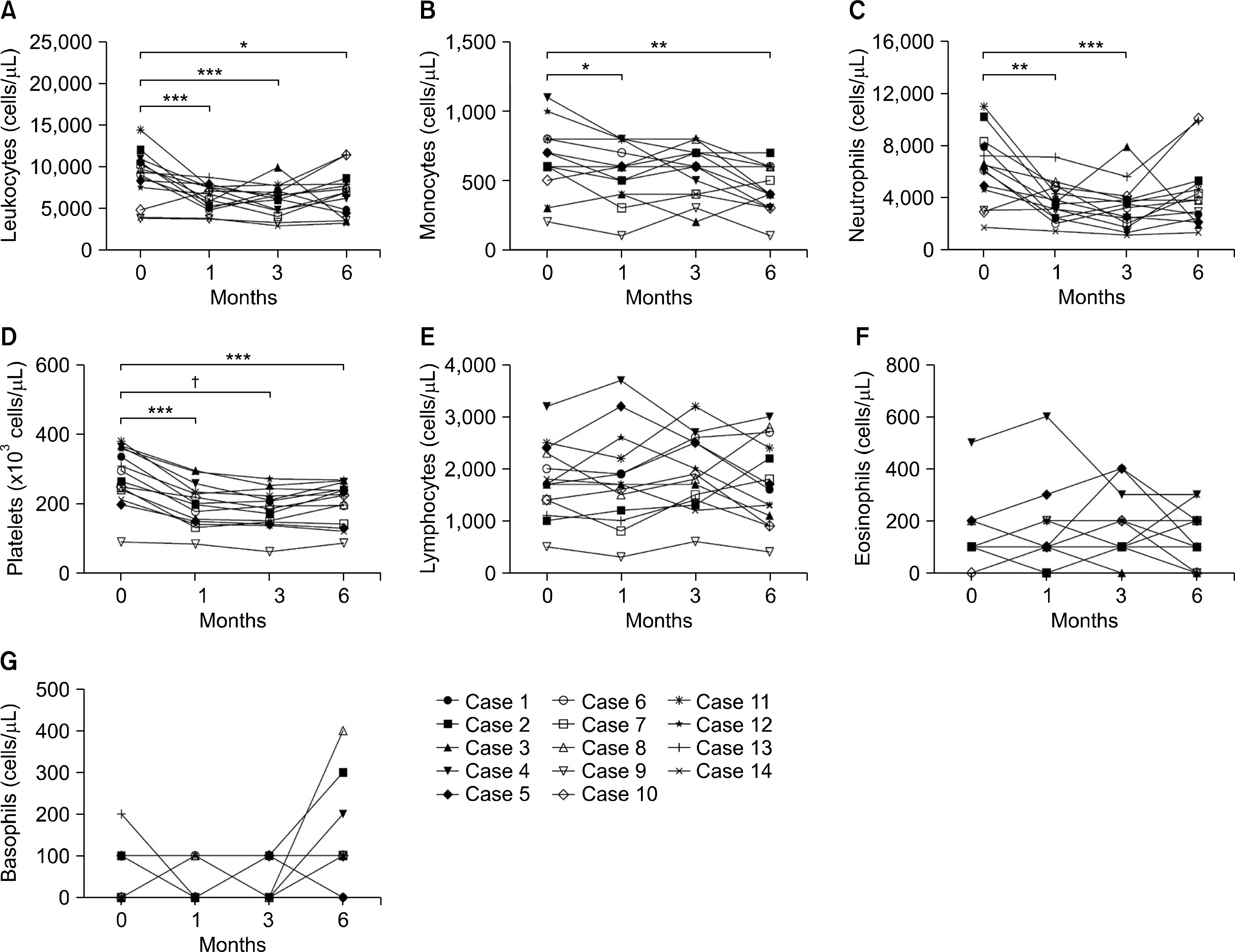 | Figure 3.Effects of tocilizumab on hematological parameters in rheumatoid arthritis (RA) patients during the six-month treatment period. Tocilizumab was administrated to 14 RA patients every four weeks. Hematologic parameters were measured at baseline and months 1, 3 and 6 during tocilizumab treatment. (A) Leukocyte count. (B) Monocyte counts. (C) Neutrophil counts. (D) Platelet counts. (E) Lymphocyte counts. (F) Eosinophil counts. (G) Basophil counts. Symbols represent individual subjects. p-values were determined using Wilcoxon's signed rank test. *p<0.05, **p<0.01, ***p<0.005, †p<0.0005. |
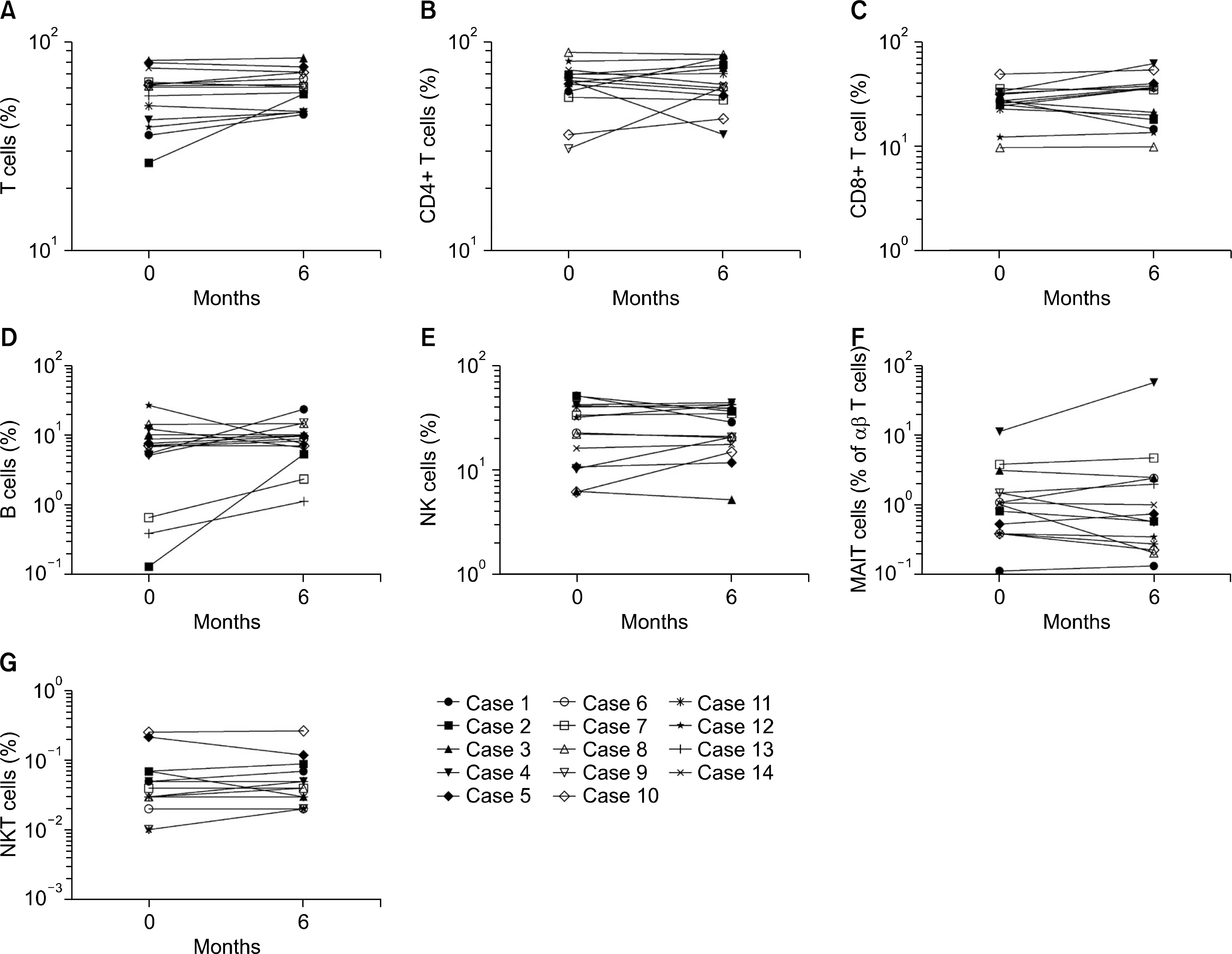 | Figure 4.Effects of tocilizumab on lymphocyte subsets in rheumatoid arthritis (RA) patients during the six-month treatment period. Tocilizumab was administrated to 14 RA patients every four weeks. Percentages of lymphocyte subsets were measured at baseline and 6 months after tocilizumab treatment. Freshly isolated peripheral blood mononuclear cells (PBMCs) from 14 RA patients were stained with appropriate monoclonal antibodies and then analyzed by flow cytometry. (A) CD3+ T cells. (B) CD4+ T cells. (C) CD8+ T cells. (D) CD3− CD19+ B cells. (E) Natural killer (NK; CD3− CD56+) cells. (F) Mucosal associated invariant T (MAIT; CD3+ TCRγδ− Vα7.2+ CD161 high) cells. (G) Natural killer T (NKT; CD3+6B11+) cells. Symbols represent individual subjects. p-values were determined using Wilcoxon's signed rank test. |
Table 1.
Baseline clinical characteristics of the 14 patients with RA
| Patient number | Age (yr)/sex | Disease duration (yr) | DAS28 | CRP (mg/dL) | ESR (mm/h) | Comorbidity | Previous medication* | Current medication |
|---|---|---|---|---|---|---|---|---|
| 1 | 61/F | 3 | 8.05 | 6.89 | 85 | None | IFX, MTX, PRD | MTX, PRD, TCZ |
| 2 | 73/F | 5 | 6.41 | 2.63 | 57 | None | MTX, RTX, PRD | MTX, PRD, TCZ |
| 3 | 37/F | 1 | 5.25 | 0.88 | 93 | None | ADA, MTX, PRD | MTX, PRD, TCZ |
| 4 | 60/F | 1 | 4.98 | 1.39 | 88 | DM, HTN | ADA, MTX, PRD | MTX, TCZ |
| 5 | 35/F | 8 | 3.63 | 0.59 | 49 | None | ETN, MTX, PRD | MTX, PRD, TCZ |
| 6 | 62/F | 16 | 4.58 | 1.42 | 70 | None | ADA, MTX | MTX, TCZ |
| 7 | 23/F | 3 | 5.36 | 3.53 | 80 | None | MTX, RTX | MTX, TCZ |
| 8 | 62/F | 10 | 5.76 | 3.60 | 104 | HTN | ETN, PRD, MTX | MTX, PRD, TCZ |
| 9 | 54/F | 8 | 5.34 | 3.50 | 41 | HTN | IFX, MTX, PRD | MTX, PRD, TCZ |
| 10 | 67/F | 15 | 5.35 | 0.23 | 46 | None | IFX, MTX, PRD | PRD, TCZ |
| 11 | 71/F | 11 | 4.72 | 2.01 | 106 | HTN | LEF, MTX, PRD | MTX, PRD, TCZ |
| 12 | 64/F | 21 | 4.85 | 1.12 | 49 | DM | ADA, MTX, PRD | MTX, PRD, TCZ |
| 13 | 39/F | 2 | 4.58 | 2.12 | 49 | None | MTX, SSZ, PRD | MTX, PRD, TCZ |
| 14 | 64/F | 8 | 4.87 | 1.49 | 65 | None | MTX, PRD, TAC | MTX, PRD, TCZ |
ADA: adalimumab, CRP: C-reactive protein, DAS28: 28-joint disease activity score, DM: diabetes mellitus, ESR: erythrocyte sedimentation rate, ETN: etanercept, F: female, HTN: hypertension, IFX: infliximab, LEF: leflunomide, MTX: methotrexate, PRD: prednisolone, RA: rheumatoid arthritis, RTX: rituximab, SSZ: sulfasalazine, TAC: tacrolimus, TCZ: tocilizumab.
Table 2.
Spearman's correlation coefficients for serum hep-cidin-25 with respect to clinical and laboratory parameters in 14 RA patients before tocilizumab therapy
Table 3.
Spearman's correlation coefficients for changes from baseline in serum hepcidin-25 with respect to changes from baseline in clinical and laboratory parameters in 14 RA patients treated with tocilizumab over 6-month treatment




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download