Abstract
Rheumatoid arthritis (RA) is an autoimmune disease that starts with decreased tolerance to modified self-antigens and eventually leads to synovitis and destruction of bone and cartilage. Age is a risk factor for developing RA. Major changes in the immune system come with age due to chronic oxidative stress on the deoxyribonucleic acid (DNA) damage pathway, somatic mutation, modifications of autoantigens, T cell tolerance and activation of fibroblast-like synoviocytes (FLS). Reactive oxygen species (ROS) generated by nicotinamide adenine dinucleotide phosphate oxidase 2 (NADPH oxidase 2) suppress T cell receptor signaling. Sirtuin 1 (SIRT1) is a critical immune suppressor of T cell activation and a key regulator of oxidative stress. When oxidative stress reduces activity of SIRT1, the breakdown of tolerance to modified self-antigens is expected. Generation of ROS can be perpetuated by enhanced DNA damage and dysfunctional mitochondria in a feedback loop during the development of RA. Through major T cell loss and selective proliferation of peripheral T cells, pro-inflammatory T cell pools with abnormal features are established in the T cell compartment. Hypoxic and inflammatory condition in synovium perpetuates ROS generation, which leads to the activation of FLS. In both T cell and synovium compartment, oxidative stress reshapes the immune system into the development of pre-clinical RA.
REFERENCES
1. Williams MS, Kwon J. T cell receptor stimulation, reactive oxygen species, and cell signaling. Free Radic Biol Med. 2004; 37:1144–51.

2. Holmström KM, Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol. 2014; 15:411–21.

3. Lambeth JD, Neish AS. Nox enzymes and new thinking on reactive oxygen: a double-edged sword revisited. Annu Rev Pathol. 2014; 9:119–45.

5. Filippin LI, Vercelino R, Marroni NP, Xavier RM. Redox signalling and the inflammatory response in rheumatoid arthritis. Clin Exp Immunol. 2008; 152:415–22.

6. Kim YS, Morgan MJ, Choksi S, Liu ZG. TNF-induced activation of the Nox1 NADPH oxidase and its role in the induction of necrotic cell death. Mol Cell. 2007; 26:675–87.

7. García-Quintans N, Prieto I, Sánchez-Ramos C, Luque A, Arza E, Olmos Y, et al. Regulation of endothelial dynamics by PGC-1α relies on ROS control of VEGF-A signaling. Free Radic Biol Med. 2016; 93:41–51.

8. Bae YS, Oh H, Rhee SG, Yoo YD. Regulation of reactive oxygen species generation in cell signaling. Mol Cells. 2011; 32:491–509.

9. Jackson SH, Devadas S, Kwon J, Pinto LA, Williams MS. T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation. Nat Immunol. 2004; 5:818–27.

10. Kwon J, Shatynski KE, Chen H, Morand S, de Deken X, Miot F, et al. The nonphagocytic NADPH oxidase Duox1 mediates a positive feedback loop during T cell receptor signaling. Sci Signal. 2010; 3:ra59.

11. Olofsson P, Holmberg J, Tordsson J, Lu S, Akerström B, Holmdahl R. Positional identification of Ncf1 as a gene that regulates arthritis severity in rats. Nat Genet. 2003; 33:25–32.

12. Jeon CH, Ahn JK, Chai JY, Kim HJ, Bae EK, Park SH, et al. Hypoxia appears at pre-arthritic stage and shows co-local-ization with early synovial inflammation in collagen induced arthritis. Clin Exp Rheumatol. 2008; 26:646–8.
13. Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000; 908:244–54.

14. Cannizzo ES, Clement CC, Sahu R, Follo C, Santambrogio L. Oxidative stress, inflamm-aging and immunosenescence. J Proteomics. 2011; 74:2313–23.

15. Xia S, Zhang X, Zheng S, Khanabdali R, Kalionis B, Wu J, et al. An update on inflamm-aging: mechanisms, prevention, and treatment. J Immunol Res. 2016; 2016; 8426874.

16. Boren E, Gershwin ME. Inflamm-aging: autoimmunity, and the immune-risk phenotype. Autoimmun Rev. 2004; 3:401–6.

17. Goronzy JJ, Shao L, Weyand CM. Immune aging and rheumatoid arthritis. Rheum Dis Clin North Am. 2010; 36:297–310.

18. Hultqvist M, Olofsson P, Holmberg J, Bäckström BT, Tordsson J, Holmdahl R. Enhanced autoimmunity, arthritis, and encephalomyelitis in mice with a reduced oxidative burst due to a mutation in the Ncf1 gene. Proc Natl Acad Sci U S A. 2004; 101:12646–51.

19. Lee K, Won HY, Bae MA, Hong JH, Hwang ES. Spontaneous and aging-dependent development of arthritis in NADPH oxidase 2 deficiency through altered differentiation of CD11b+ and Th/Treg cells. Proc Natl Acad Sci U S A. 2011; 108:9548–53.

20. Shah D, Wanchu A, Bhatnagar A. Interaction between oxidative stress and chemokines: possible pathogenic role in systemic lupus erythematosus and rheumatoid arthritis. Immunobiology. 2011; 216:1010–7.

21. Henrotin YE, Bruckner P, Pujol JP. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthritis Cartilage. 2003; 11:747–55.

22. Garrett IR, Boyce BF, Oreffo RO, Bonewald L, Poser J, Mundy GR. Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Invest. 1990; 85:632–9.

23. Biniecka M, Connolly M, Gao W, Ng CT, Balogh E, Gogarty M, et al. Redox-mediated angiogenesis in the hypoxic joint of inflammatory arthritis. Arthritis Rheumatol. 2014; 66:3300–10.

24. Tak PP, Zvaifler NJ, Green DR, Firestein GS. Rheumatoid arthritis and p53: how oxidative stress might alter the course of inflammatory diseases. Immunol Today. 2000; 21:78–82.

25. Yamanishi Y, Boyle DL, Green DR, Keystone EC, Connor A, Zollman S, et al. p53 tumor suppressor gene mutations in fibroblast-like synoviocytes from erosion synovium and non-erosion synovium in rheumatoid arthritis. Arthritis Res Ther. 2005; 7:R12–8.

26. Biniecka M, Fox E, Gao W, Ng CT, Veale DJ, Fearon U, et al. Hypoxia induces mitochondrial mutagenesis and dysfunction in inflammatory arthritis. Arthritis Rheum. 2011; 63:2172–82.

27. Weyand CM, Fujii H, Shao L, Goronzy JJ. Rejuvenating the immune system in rheumatoid arthritis. Nat Rev Rheumatol. 2009; 5:583–8.

28. Rane S, Das R, Ranganathan V, Prabhu S, Das A, Mattoo H, et al. Peripheral residence of naïve CD4 T cells induces MHC class II-dependent alterations in phenotype and function. BMC Biol. 2014; 12:106.

29. Shao L, Fujii H, Colmegna I, Oishi H, Goronzy JJ, Weyand CM. Deficiency of the DNA repair enzyme ATM in rheumatoid arthritis. J Exp Med. 2009; 206:1435–49.

30. Ponchel F, Morgan AW, Bingham SJ, Quinn M, Buch M, Verburg RJ, et al. Dysregulated lymphocyte proliferation and differentiation in patients with rheumatoid arthritis. Blood. 2002; 100:4550–6.

31. Ryan BJ, Nissim A, Winyard PG. Oxidative post-translational modifications and their involvement in the pathogenesis of autoimmune diseases. Redox Biol. 2014; 2:715–24.

32. Quiñonez-Flores CM, González-Chávez SA, Del Río Nájera D, Pacheco-Tena C. Oxidative stress relevance in the pathogenesis of the rheumatoid arthritis: a systematic review. Biomed Res Int. 2016; 2016; 6097417.

33. Strollo R, Ponchel F, Malmström V, Rizzo P, Bombardieri M, Wenham CY, et al. Autoantibodies to posttranslationally modified type II collagen as potential biomarkers for rheumatoid arthritis. Arthritis Rheum. 2013; 65:1702–12.

34. Eggleton P, Nissim A, Ryan BJ, Whiteman M, Winyard PG. Detection and isolation of human serum autoantibodies that recognize oxidatively modified autoantigens. Free Radic Biol Med. 2013; 57:79–91.

36. de Groot L, Hinkema H, Westra J, Smit AJ, Kallenberg CG, Bijl M, et al. Advanced glycation endproducts are increased in rheumatoid arthritis patients with controlled disease. Arthritis Res Ther. 2011; 13:R205.

37. Zheng S, Zhong ZM, Qin S, Chen GX, Wu Q, Zeng JH, et al. Advanced oxidation protein products induce inflammatory response in fibroblast-like synoviocytes through NADPH oxidase-dependent activation of NF-κ B. Cell Physiol Biochem. 2013; 32:972–85.
38. Steenvoorden MM, van der Helm-van Mil AH, Stoeken G, Bank RA, Devries RR, Huizinga TW, et al. The RAGE G82S polymorphism is not associated with rheumatoid arthritis independently of HLA-DRB1*0401. Rheumatology (Oxford). 2006; 45:488–90.

39. Takahashi T, Katsuta S, Tamura Y, Nagase N, Suzuki K, Nomura M, et al. Bone-targeting endogenous secretory receptor for advanced glycation end products rescues rheumatoid arthritis. Mol Med. 2013; 19:183–94.

40. Sena LA, Li S, Jairaman A, Prakriya M, Ezponda T, Hildeman DA, et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity. 2013; 38:225–36.

41. Gelderman KA, Hultqvist M, Pizzolla A, Zhao M, Nandakumar KS, Mattsson R, et al. Macrophages suppress T cell responses and arthritis development in mice by producing reactive oxygen species. J Clin Invest. 2007; 117:3020–8.

42. King MR, Ismail AS, Davis LS, Karp DR. Oxidative stress promotes polarization of human T cell differentiation toward a T helper 2 phenotype. J Immunol. 2006; 176:2765–72.

43. Peterson JD, Herzenberg LA, Vasquez K, Waltenbaugh C. Glutathione levels in antigen-presenting cells modulate Th1 versus Th2 response patterns. Proc Natl Acad Sci U S A. 1998; 95:3071–6.

44. Zhi L, Ustyugova IV, Chen X, Zhang Q, Wu MX. Enhanced Th17 differentiation and aggravated arthritis in IEX-1-defi-cient mice by mitochondrial reactive oxygen species-mediated signaling. J Immunol. 2012; 189:1639–47.

45. Yang Z, Shen Y, Oishi H, Matteson EL, Tian L, Goronzy JJ, et al. Restoring oxidant signaling suppresses proarthrito-genic T cell effector functions in rheumatoid arthritis. Sci Transl Med. 2016; 8:331ra38.

46. Nefla M, Holzinger D, Berenbaum F, Jacques C. The danger from within: alarmins in arthritis. Nat Rev Rheumatol. 2016; 12:669–83.

47. Kerkhoff C, Nacken W, Benedyk M, Dagher MC, Sopalla C, Doussiere J. The arachidonic acid-binding protein S100A8/ A9 promotes NADPH oxidase activation by interaction with p67phox and Rac-2. FASEB J. 2005; 19:467–9.
48. Green DR, Galluzzi L, Kroemer G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science. 2011; 333:1109–12.

50. Salminen A, Ojala J, Kaarniranta K, Kauppinen A. Mitochondrial dysfunction and oxidative stress activate inflammasomes: impact on the aging process and age-related diseases. Cell Mol Life Sci. 2012; 69:2999–3013.

51. Li ZC, Xiao J, Peng JL, Chen JW, Ma T, Cheng GQ, et al. Functional annotation of rheumatoid arthritis and osteoarthritis associated genes by integrative genomewide gene expression profiling analysis. PLoS One. 2014; 9:e85784.

52. Helenius M, Kyrylenko S, Vehviläinen P, Salminen A. Characterization of aging-associated upregulation of constitutive nuclear factor-kappa B binding activity. Antioxid Redox Signal. 2001; 3:147–56.
53. Anrather J, Racchumi G, Iadecola C. NF-kappaB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J Biol Chem. 2006; 281:5657–67.
54. Rajendran R, Garva R, Krstic-Demonacos M, Demonacos C. Sirtuins: molecular traffic lights in the crossroad of oxidative stress, chromatin remodeling, and transcription. J Biomed Biotechnol. 2011; 2011; 368276.

55. Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004; 303:2011–5.

56. Salminen A, Kaarniranta K. SIRT1: regulation of longevity via autophagy Cell Signal. 2009; 21:1356–60.
57. Rajendrasozhan S, Yang SR, Kinnula VL, Rahman I. SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008; 177:861–70.

58. Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, et al. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci U S A. 2008; 105:3374–9.

59. Sengupta A, Molkentin JD, Paik JH, DePinho RA, Yutzey KE. FoxO transcription factors promote cardiomyocyte survival upon induction of oxidative stress. J Biol Chem. 2011; 286:7468–78.

60. Salminen A, Kaarniranta K, Kauppinen A. Inflammaging: disturbed interplay between autophagy and inflammasomes. Aging (Albany NY). 2012; 4:166–75.

61. Niederer F, Ospelt C, Brentano F, Hottiger MO, Gay RE, Gay S, et al. SIRT1 overexpression in the rheumatoid arthritis synovium contributes to proinflammatory cytokine production and apoptosis resistance. Ann Rheum Dis. 2011; 70:1866–73.

62. Zhang J, Lee SM, Shannon S, Gao B, Chen W, Chen A, et al. The type III histone deacetylase Sirt1 is essential for maintenance of T cell tolerance in mice. J Clin Invest. 2009; 119:3048–58.

63. Elmali N, Baysal O, Harma A, Esenkaya I, Mizrak B. Effects of resveratrol in inflammatory arthritis. Inflammation. 2007; 30:1–6.

64. Xuzhu G, Komai-Koma M, Leung BP, Howe HS, McSharry C, McInnes IB, et al. Resveratrol modulates murine collagen-induced arthritis by inhibiting Th17 and B-cell function. Ann Rheum Dis. 2012; 71:129–35.

65. Shakibaei M, Buhrmann C, Mobasheri A. Resveratrol-mediated SIRT-1 interactions with p300 modulate receptor activator of NF-kappaB ligand (RANKL) activation of NF-kappaB signaling and inhibit osteoclastogenesis in bone-derived cells. J Biol Chem. 2011; 286:11492–505.
66. Kokkonen H, Söderström I, Rocklöv J, Hallmans G, Lejon K, Rantapää Dahlqvist S. Up-regulation of cytokines and chemokines predates the onset of rheumatoid arthritis. Arthritis Rheum. 2010; 62:383–91.

67. van den Berg WB. Lessons from animal models of arthritis over the past decade. Arthritis Res Ther. 2009; 11:250.

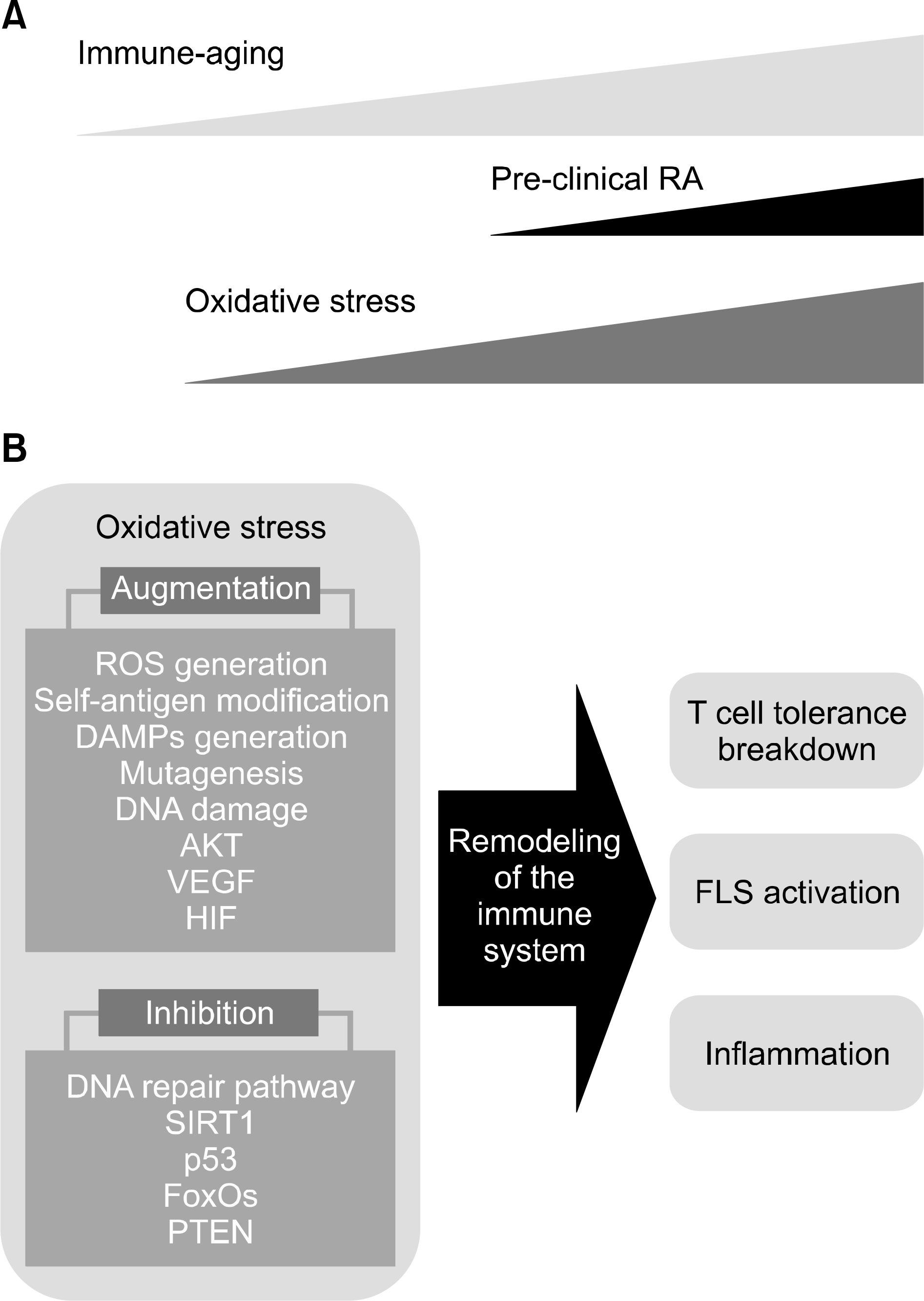
Figure 1.
Role of oxidative stress in the pathogenesis of rheumatoid arthritis (RA). (A) With advancing age, oxidative stress plays critical roles in the generation of pre-clinical RA in the process of the immune-aging. (B) Important pathways regulated by the oxidative stress in the immune-aging process re-shape the immune systems to lead breakdown of T cell tolerance, activation of fibroblast-like synoviocytes (FLS) and the generation of the inflammatory networks for pathogenesis of RA. Oxidative stress could contribute to development of RA in several ways. Enhanced generation of reactive oxygen species (ROS), oxidative modification of self-antigens, generation of danger-associated molecular patterns (DAMPs), mutagenesis of genomic deoxyribonucleic acid (DNA) and mitochondrial DNA, dysfunctional mitochondria, reduced autophagy, DNA damage, defects in DNA damage pathways, reduced activity of sirtuin 1 (SIRT1), p53, forkhead box O (FoxOs), and antioxidants, enhanced activity of AKT, activation of vascular endothelial growth factor (VEGF) and hypoxia-inducible factor (HIF) pathway and inhibition of phosphatase and tensin homo-log (PTEN).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download