Abstract
A 50-year-old woman, who had been treated for rheumatoid arthritis (RA) over a 10-year period, suddenly presented with mon-ocular vision loss while the RA had a stable course over many years. She was diagnosed with central retinal artery occlusion (CRAO) based on ophthalmologic examinations including optical coherence tomography and fluorescein angiography. There was no evidence of atherosclerosis, infection, and malignancy that can cause CRAO. Considering the association between CRAO and other rheumatic diseases, such as systemic vasculitis and systemic lupus erythematous in previous reports, it was presumed that her RA might have contributed to the development of CRAO. Although cases of CRAO in patients with RA are extremely rare, these findings suggest that physicians need to be aware of the possibility of CRAO in patients with RA who experience decreased visual acuity.
REFERENCES
1. Callizo J, Feltgen N, Pantenburg S, Wolf A, Neubauer AS, Jurklies B, et al. European Assessment Group for Lysis in the Eye. Cardiovascular risk factors in central retinal artery occlusion: results of a prospective and standardized medical examination. Ophthalmology. 2015; 122:1881–8.
2. Cugati S, Varma DD, Chen CS, Lee AW. Treatment options for central retinal artery occlusion. Curr Treat Options Neurol. 2013; 15:63–77.

3. Perumal B, Black EH, Levin F, Servat JJ. Non-infectious orbital vasculitides. Eye (Lond). 2012; 26:630–9.

4. Artifoni M, Rothschild PR, Brézin A, Guillevin L, Puéchal X. Ocular inflammatory diseases associated with rheumatoid arthritis. Nat Rev Rheumatol. 2014; 10:108–16.

5. Tong L, Thumboo J, Tan YK, Wong TY, Albani S. The eye: a window of opportunity in rheumatoid arthritis? Nat Rev Rheumatol. 2014; 10:552–60.

6. Varma DD, Cugati S, Lee AW, Chen CS. A review of central retinal artery occlusion: clinical presentation and management. Eye (Lond). 2013; 27:688–97.

7. Park SJ, Choi NK, Seo KH, Park KH, Woo SJ. Nationwide incidence of clinically diagnosed central retinal artery occlusion in Korea, 2008 to 2011. Ophthalmology. 2014; 121:1933–8.

8. Matsuo T. Multiple occlusive retinal arteritis in both eyes of a patient with rheumatoid arthritis. Jpn J Ophthalmol. 2001; 45:662–4.

9. Kachmaryk MM, Trimble SN, Gieser RG. Cilioretinal artery occlusion in sickle cell trait and rheumatoid arthritis. Retina. 1995; 15:501–4.

10. Kim SS, Lee JH, Nam TS, Kim SI. A case of polyarteritis nodosa associated with central retinal artery occlusion. J Korean Rheum Assoc. 2002; 9:236–40.
11. Hwang HS, Kang S. Combined central retinal vein and artery occlusion in systemic lupus erythematosus patient. Retin Cases Brief Rep. 2012; 6:187–8.

12. Song YH, Kim CG, Kim SD, Kim YY, Choe JY. Systemic lupus erythematosus presenting earlier as retinal vasoocclusion. Korean J Intern Med. 2001; 16:210–3.
13. Rekhi GS, Dheer S. Oral contraceptive-induced central retinal artery occlusion. J Assoc Physicians India. 2002; 50:1084–5.
Figure 1.
Clinical course of the patient during her 10-year follow-up period. CRAO: central retinal artery occlusion, CRP: C-re-active protein, ESR: erythrocyte sedimentation rate.
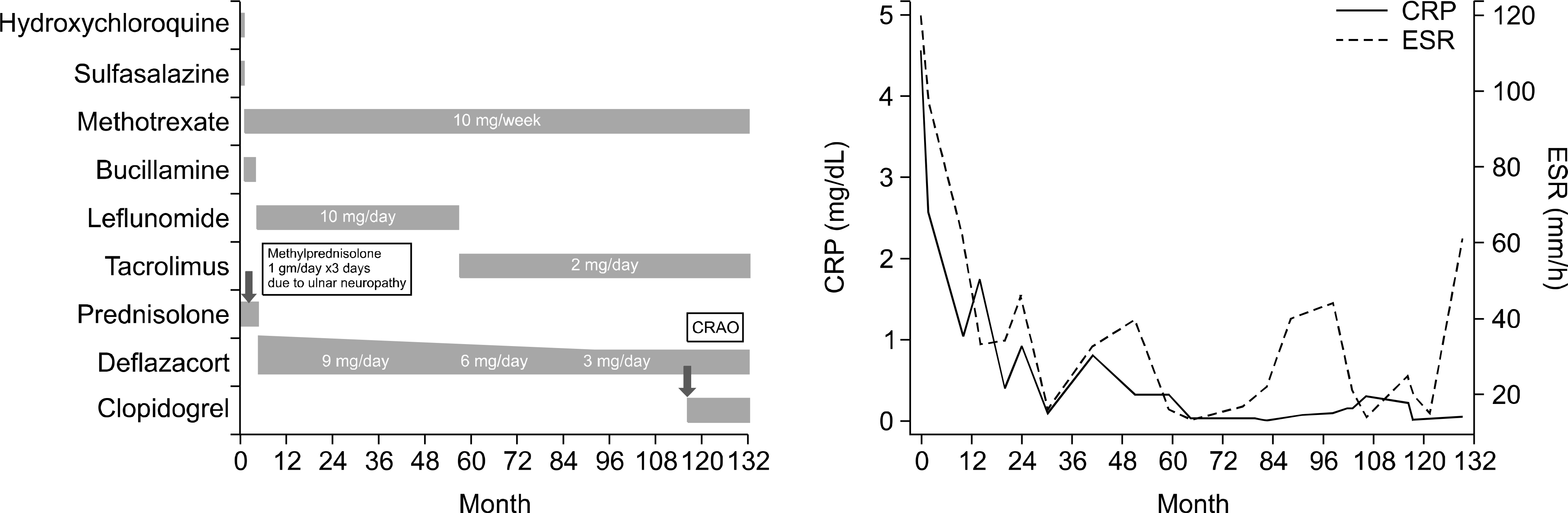
Figure 2.
Fundoscopic findings. (A) A photograph of the right eye's fundus reveals no specific findings. (B) A photograph of the left eye's fundus reveals acute central retinal artery occlusion with cherry-red spots that were caused by a retinal infarction and arte-riole cattle trucking. No definite emboli were observed.
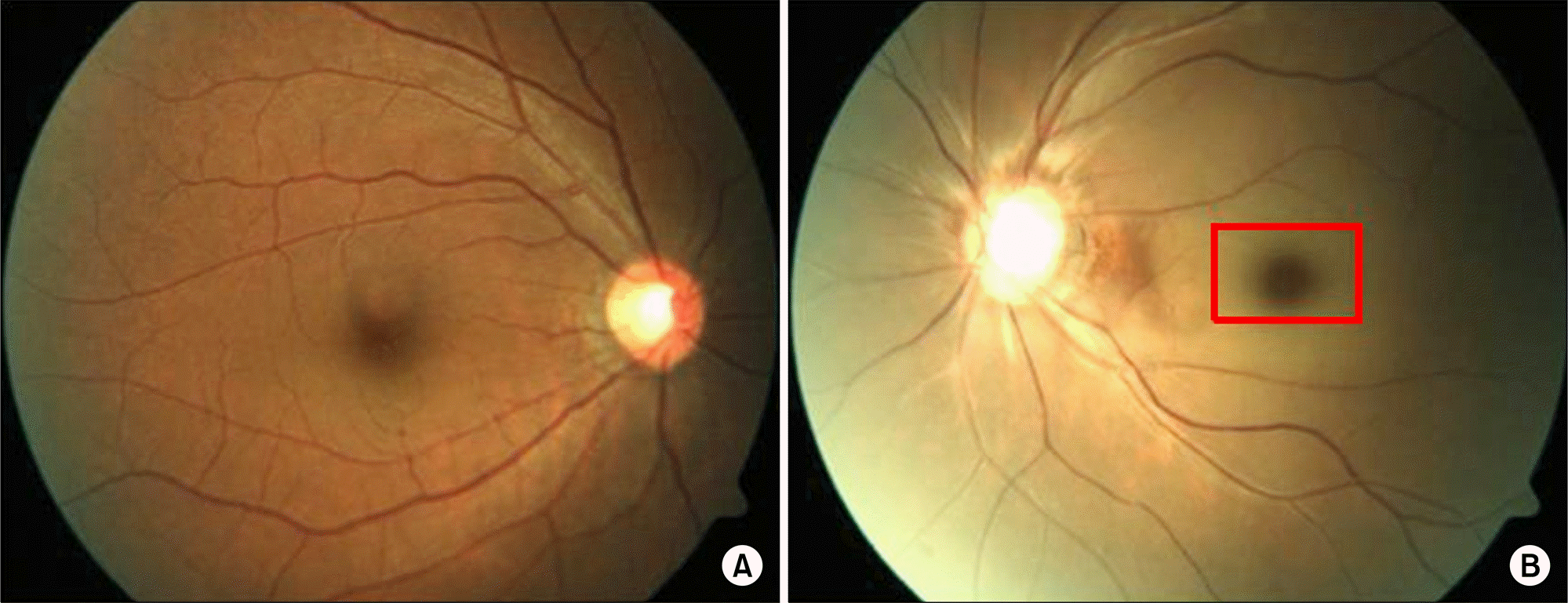
Figure 3.
Optical coherence tomography (OCT) shows cross-section images of retina. (A) OCT of the right eye shows normal appearance. (B) OCT of the left eye shows the inner retina layer (nerve fiber layer) infarct presented as thickening and whitening (red arrows).
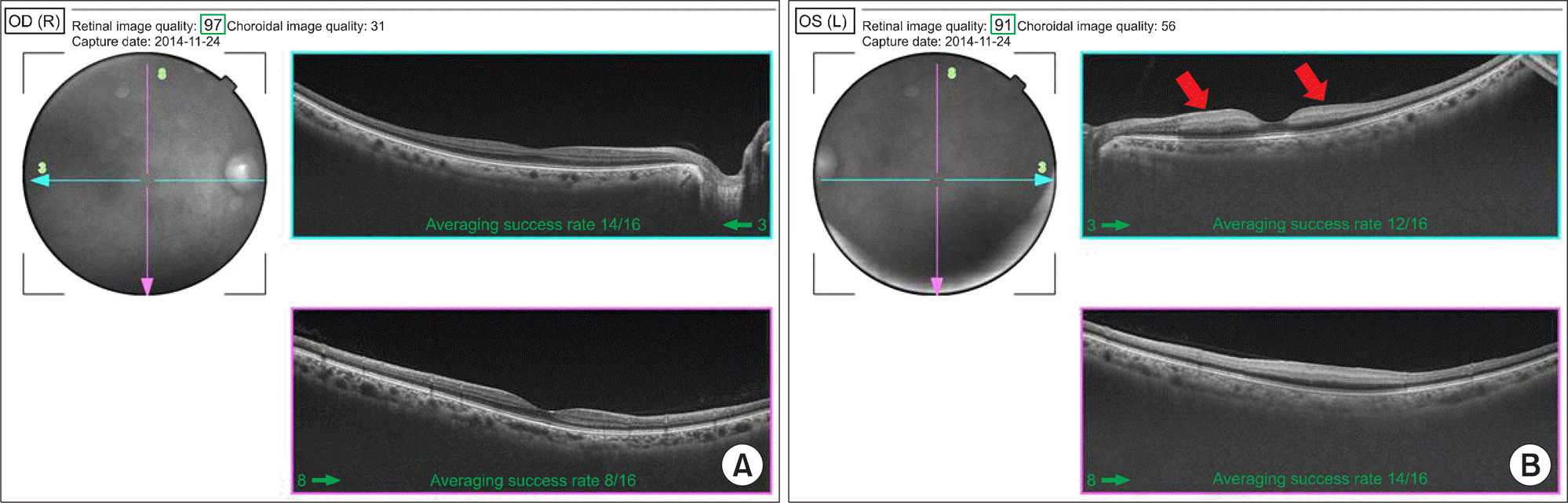
Figure 4.
Fluorescein angiography at the onset of the central retinal artery occlusion. (A) Arterial filling in the left eye was markedly delayed in the early phase. (B) The arteriovenous transit time was prolonged with no venous flow in the middle phase. (C) Fluorescein pooling is visible in the late phase.

Table 1.
Cases of central retinal artery occlusion in rheumatoid arthritis




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download