Abstract
Developments in our comprehension of the autoimmune and inflammation mechanisms in rheumatoid arthritis (RA) have produced targeted therapies that block aberrant immune cells and cytokine networks, and improved treatment of RA patients considerably. Nevertheless, limitations of these treatments include incomplete treatment response, adverse effects requiring drug withdrawal, and refractory cases. Hence, many researchers have redirected efforts towards investigation of other biological aspects of RA, including the mechanisms driving joint tissue repair and balanced immune regulation. This investigation focuses on mesenchymal stem cell (MSC) research, with the ultimate goal of developing interventions for immune modulation and repair of damaged joints. MSCs are multipotent cells capable of differentiating into mesodermal lineage cells. These cells have also attracted interest for their anti-inflammatory and immunomodulatory capacities. They have many distinctive immunological properties, inhibiting the proliferation and production of cytokines by T, B, natural killer, and dendritic cells. Indeed, MSCs have the capacity to regulate immunity-induced peripheral tolerance, suggesting they can be used as therapeutic tools in RA. This review discusses properties of MSCs, in vitro studies, animal studies, and clinical trials involving MSCs. Our review discusses the current knowledge of the mechanisms of MSC-mediated immunosuppression and potential therapeutic uses of MSCs in RA.
REFERENCES
2. Gonzalez A, Maradit Kremers H, Crowson CS, Nicola PJ, Davis JM 3rd, Therneau TM, et al. The widening mortality gap between rheumatoid arthritis patients and the general population. Arthritis Rheum. 2007; 56:3583–7.

4. Espinoza F, Fabre S, Pers YM. Remission-induction therapies for early rheumatoid arthritis: evidence to date and clinical implications. Ther Adv Musculoskelet Dis. 2016; 8:107–18.

5. Cohen G, Gossec L, Dougados M, Cantagrel A, Goupille P, Daures JP, et al. Radiological damage in patients with rheumatoid arthritis on sustained remission. Ann Rheum Dis. 2007; 66:358–63.

6. Smolen JS, Aletaha D, Koeller M, Weisman MH, Emery P. New therapies for treatment of rheumatoid arthritis. Lancet. 2007; 370:1861–74.

7. Chiang EY, Kolumam GA, Yu X, Francesco M, Ivelja S, Peng I, et al. Targeted depletion of lymphotoxin-alpha-expressing TH1 and TH17 cells inhibits autoimmune disease. Nat Med. 2009; 15:766–73.
8. Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, et al. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004; 200:277–85.
9. Tyndall A, van Laar JM. Stem cells in the treatment of inflammatory arthritis. Best Pract Res Clin Rheumatol. 2010; 24:565–74.

10. Liang J, Li X, Zhang H, Wang D, Feng X, Wang H, et al. Allogeneic mesenchymal stem cells transplantation in patients with refractory RA. Clin Rheumatol. 2012; 31:157–61.

11. Wang L, Wang L, Cong X, Liu G, Zhou J, Bai B, et al. Human umbilical cord mesenchymal stem cell therapy for patients with active rheumatoid arthritis: safety and efficacy. Stem Cells Dev. 2013; 22:3192–202.

12. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284:143–7.

13. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006; 8:315–7.

14. Tyndall A, LeBlanc K. Stem cells and rheumatology: update on adult stem cell therapy in autoimmune diseases. Arthritis Rheum. 2006; 55:521–5.

15. Sotiropoulou PA, Perez SA, Salagianni M, Baxevanis CN, Papamichail M. Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells. 2006; 24:462–71.

16. Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringdén O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003; 31:890–6.
17. Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003; 75:389–97.

18. Eliopoulos N, Stagg J, Lejeune L, Pommey S, Galipeau J. Allogeneic marrow stromal cells are immune rejected by MHC class I- and class II-mismatched recipient mice. Blood. 2005; 106:4057–65.

19. Nauta AJ, Westerhuis G, Kruisselbrink AB, Lurvink EG, Willemze R, Fibbe WE. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood. 2006; 108:2114–20.

20. Prigozhina TB, Khitrin S, Elkin G, Eizik O, Morecki S, Slavin S. Mesenchymal stromal cells lose their immunosuppressive potential after allotransplantation. Exp Hematol. 2008; 36:1370–6.

21. Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008; 2:141–50.

22. Dazzi F, Krampera M. Mesenchymal stem cells and autoimmune diseases. Best Pract Res Clin Haematol. 2011; 24:49–57.

23. Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004; 103:4619–21.

24. Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, et al. Bone marrow stromal cells attenu-ate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009; 15:42–9.

25. Asari S, Itakura S, Ferreri K, Liu CP, Kuroda Y, Kandeel F, et al. Mesenchymal stem cells suppress B-cell terminal differentiation. Exp Hematol. 2009; 37:604–15.

26. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005; 105:1815–22.

27. Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008; 26:212–22.

28. Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005; 105:2821–7.

29. Rasmusson I, Ringdén O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. 2003; 76:1208–13.

30. Prevosto C, Zancolli M, Canevali P, Zocchi MR, Poggi A. Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell-lymphocyte interaction. Haematologica. 2007; 92:881–8.

31. Tabera S, Pérez-Simón JA, Díez-Campelo M, Sánchez-Abarca LI, Blanco B, López A, et al. The effect of mesenchymal stem cells on the viability, proliferation and differentiation of B-lymphocytes. Haematologica. 2008; 93:1301–9.

32. Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008; 111:1327–33.

33. Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol. 2006; 177:2080–7.

34. Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006; 24:74–85.

35. Sordi V, Malosio ML, Marchesi F, Mercalli A, Melzi R, Giordano T, et al. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood. 2005; 106:419–27.

36. Stappenbeck TS, Miyoshi H. The role of stromal stem cells in tissue regeneration and wound repair. Science. 2009; 324:1666–9.

37. Leibacher J, Henschler R. Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem Cell Res Ther. 2016; 7:7.

38. Augello A, Tasso R, Negrini SM, Cancedda R, Pennesi G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum. 2007; 56:1175–86.

39. González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum. 2009; 60:1006–19.

40. Choi JJ, Yoo SA, Park SJ, Kang YJ, Kim WU, Oh IH, et al. Mesenchymal stem cells overexpressing interleukin-10 at-tenuate collagen-induced arthritis in mice. Clin Exp Immunol. 2008; 153:269–76.

41. Park MJ, Park HS, Cho ML, Oh HJ, Cho YG, Min SY, et al. Transforming growth factor β-transduced mesenchymal stem cells ameliorate experimental autoimmune arthritis through reciprocal regulation of Treg/Th17 cells and osteoclastogenesis. Arthritis Rheum. 2011; 63:1668–80.

42. Liu Y, Mu R, Wang S, Long L, Liu X, Li R, et al. Therapeutic potential of human umbilical cord mesenchymal stem cells in the treatment of rheumatoid arthritis. Arthritis Res Ther. 2010; 12:R210.

43. Bouffi C, Bony C, Courties G, Jorgensen C, Noël D. IL-6-de-pendent PGE2 secretion by mesenchymal stem cells inhibits local inflammation in experimental arthritis. PLoS One. 2010; 5:e14247.

44. Djouad F, Fritz V, Apparailly F, Louis-Plence P, Bony C, Sany J, et al. Reversal of the immunosuppressive properties of mesenchymal stem cells by tumor necrosis factor alpha in collagen-induced arthritis. Arthritis Rheum. 2005; 52:1595–603.
45. Chen B, Hu J, Liao L, Sun Z, Han Q, Song Z, et al. Flk-1+ mesenchymal stem cells aggravate collagen-induced arthritis by upregulating interleukin-6. Clin Exp Immunol. 2010; 159:292–302.

46. Schurgers E, Kelchtermans H, Mitera T, Geboes L, Matthys P. Discrepancy between the in vitro and in vivo effects of murine mesenchymal stem cells on T-cell proliferation and collagen-induced arthritis. Arthritis Res Ther. 2010; 12:R31.
47. MacDonald GI, Augello A, De Bari C. Role of mesenchymal stem cells in reestablishing immunologic tolerance in autoimmune rheumatic diseases. Arthritis Rheum. 2011; 63:2547–57.

48. Meyerrose TE, De Ugarte DA, Hofling AA, Herrbrich PE, Cordonnier TD, Shultz LD, et al. In vivo distribution of human adipose-derived mesenchymal stem cells in novel xen-otransplantation models. Stem Cells. 2007; 25:220–7.

49. Park KH, Mun CH, Kang MI, Lee SW, Lee SK, Park YB. Treatment of collagen-induced arthritis using immune modulatory properties of human mesenchymal stem cells. Cell Transplant. 2016; 25:1057–72.

50. Garimella MG, Kour S, Piprode V, Mittal M, Kumar A, Rani L, et al. Adipose-derived mesenchymal stem cells prevent systemic bone loss in collagen-induced arthritis. J Immunol. 2015; 195:5136–48.

51. Ra JC, Kang SK, Shin IS, Park HG, Joo SA, Kim JG, et al. Stem cell treatment for patients with autoimmune disease by systemic infusion of culture-expanded autologous adipose tissue derived mesenchymal stem cells. J Transl Med. 2011; 9:181.

52. Safety and efficacy study of human umbilical cord-mesenchymal stem cells for rheumatoid arthritis. Silver Spring (MD): U.S. Food and Drug Administration;2016 Jan. ClinicalTrials.gov Identifier: NCT02643823. Available from:. https://clinicaltrials.gov/ct2/show/NCT02643823?term=NCT02643823&rank=1.
53. Feasibility study of umbilical cord tissue drived mesenchymal stem cells (UC-MSC) in disease modifying antirheumatic drugs (DMARD) resistant rheumatoid arthritis. Panama: Panama: Ministry of Health;. 2013; Oct. ClinicalTrials.gov Identifier: NCT01985464. Available from:. https://clinicaltrials.gov/ct2/show/NCT01985464?term=NCT01985464&rank=1.
54. Phase Ib/IIa, Escalating dose, single blind, clinical trial to assess the safety of the i.v administration of allogeneic adi-pose-derived mesenchymal cells (eASCs) to refractory rheumatoid arthritis patients. Madrid: Spanish Agency for Medicines and Health Products;. 2011; Aug. ClinicalTrials. gov Identifier: NCT01663116. Available from:. https://clinicaltrials.gov/ct2/show/NCT01663116?term=NCT01663116&rank=1.
Figure 1.
Suppressive effects of MSCs on immune cells. The effects of MSCs on cells of the immune system are anti-inflammatory. DCs: dendritic cells, MSCs: mesenchymal stem cells, NK cell: natural killer cell, TREG cell: regulatory T cell.
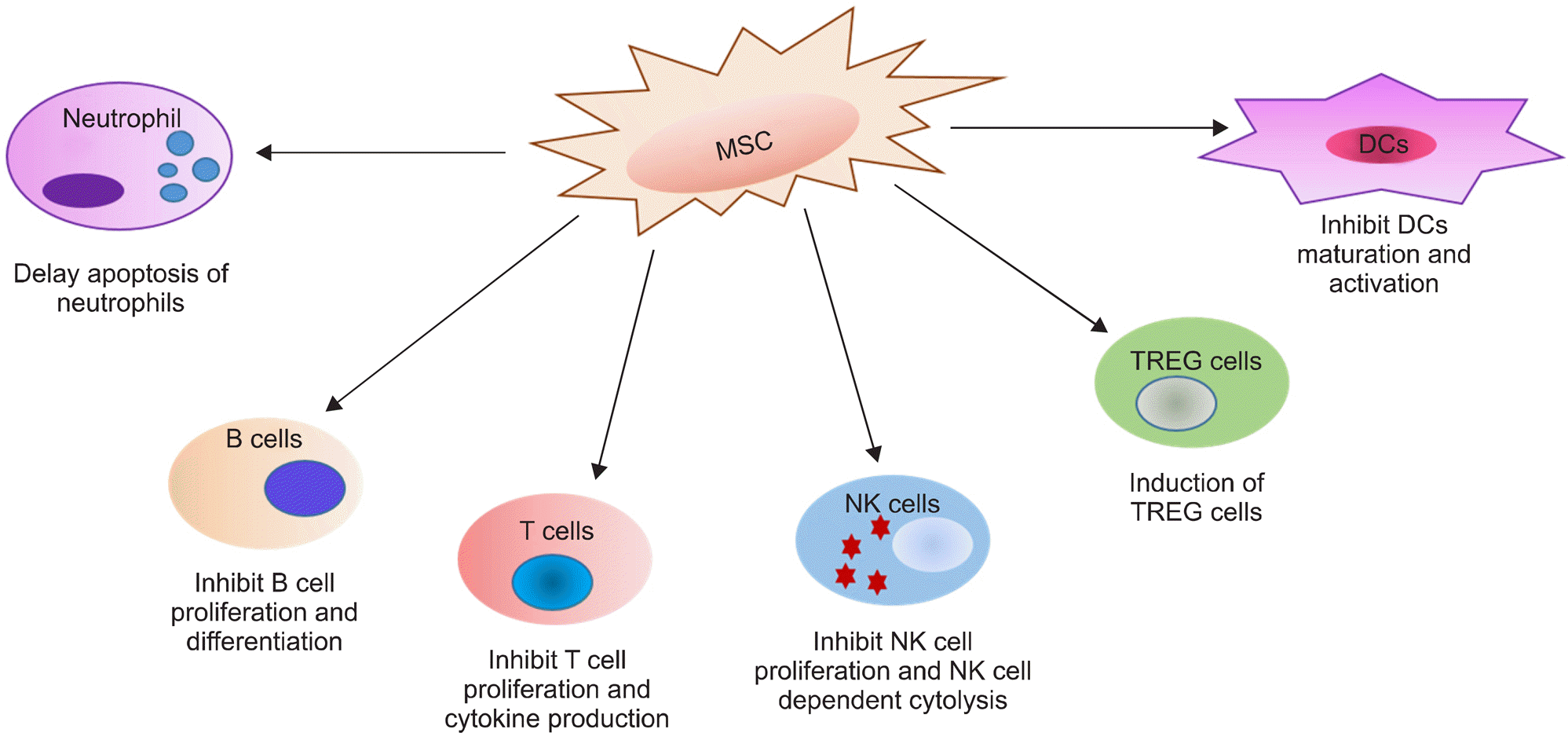
Table 1.
The literature describing the effects of mesenchymal stem cells (MSCs) in mouse model of rheumatoid arthritis
| First author, year [ref] | Source of MSC D | Donor– recipient MHC match | Dose of MSCs | s Route of administration | Time of treatment | Outcome of treatment |
|---|---|---|---|---|---|---|
| Djouad et al.,2005 [44] | C3 mouse cell line | Allogeneic | 1×106, 1×106, 4×106 | Intravenous | Day 0 or 21, day 0 or 21 | UA* |
| Augello et al.,2007 [38] | Mouse BM | Allogeneic | 5×106 | Intraperitoneal | Day 0 or 21 | Positive† |
| Gonzalez et al.,2009 [39] | Adipose tissue: human, mouse | Xenogeneic, allogeneic | 1×106 | Intraperitoneal, intraarticular | Once per day for 5 days ADO | Positive† |
| Choi et al.,2008 [40] | Mouse BM | Syngeneic | 1×106 | Intravenous | Day 21, 28, 35 | Positive† |
| Park et al.,2011 [41] | Mouse BM | Syngeneic | 1×106 | Intraperitoneal | Week 7 | Positive† |
| Liu et al.,2010 [42] | Human UC | Xenogeneic | 5×106 | Intraperitoneal | Day 31 ADO | Positive† |
| Bouffi et al.,2010 [43] | Mouse BM | Syngeneic, allogeneic | 1×106 | Intravenous | Day 18, 24,28, 32 | Positive† |
| Chen et al.,2010 [45] | Mouse BM | Syngeneic | 1×106, 2×106 | Intravenous | Day 0 or 21 | Negative‡ |
| Schurgers et al.,2010 [46] | Mouse BM | Syngeneic, allogeneic | 1×106 | Intravenous, intraperitoneal | Day 1, 16,19, 23, 30 | Negative‡ |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download