Abstract
Gitelman's syndrome (GS), a hereditary disease characterized by hypokalemia, hypomagnesemia, and hypocalciuria, is a salt-losing renal tubulopathy. Herein, we describe a case of a 28-year-old woman diagnosed with atypical GS accompanying chondrocalcinosis. One year ago, she presented with vomiting, hypokalemic metabolic alkalosis, and hypocalciuria, and was tested by diuretic challenge test. As a result, she was diagnosed with atypical GS with normomagnesemia and treated with spi-ronolactone and potassium supplementation. Meanwhile, acute arthritis of the right 1st metatarsophalangeal joint occurred. On the radiographies of the knees, chondrocalcinosis was observed. To the best of our knowledge, this is the first report in Korea of GS with chondrocalcinosis. Antialdosterone therapy or magnesium supplementation is effective in preventing the progression of chondrocalcinosis; thus, early diagnosis and treatment of GS are important.
Go to : 
REFERENCES
1. Gitelman HJ, Graham JB, Welt LG. A new familial disorder characterized by hypokalemia and hypomagnesemia. Trans Assoc Am Physicians. 1966; 79:221–35.
2. Bettinelli A, Bianchetti MG, Girardin E, Caringella A, Cecconi M, Appiani AC, et al. Use of calcium excretion values to distinguish two forms of primary renal tubular hypokalemic alkalosis: Bartter and Gitelman syndromes. J Pediatr. 1992; 120:38–43.

4. Sanmartí R, Pañella D, Brancós MA, Canela J, Collado A, Brugués J. Prevalence of articular chondrocalcinosis in elderly subjects in a rural area of Catalonia. Ann Rheum Dis. 1993; 52:418–22.
5. Koçkara AŞ, Candan F, Hüzmeli C, Kayataş M, Alaygut D. Gitelman's syndrome associated with chondrocalcinosis: a case report. Ren Fail. 2013; 35:1285–8.

6. Hisakawa N, Yasuoka N, Itoh H, Takao T, Jinnouchi C, Nishiya K, et al. A case of Gitelman's syndrome with chondrocalcinosis. Endocr J. 1998; 45:261–7.

7. Vargas-Poussou R, Dahan K, Kahila D, Venisse A, Riveira-Munoz E, Debaix H, et al. Spectrum of mutations in Gitelman syndrome. J Am Soc Nephrol. 2011; 22:693–703.

8. Nakamura A, Shimizu C, Nagai S, Yoshida M, Aoki K, Kondo T, et al. Problems in diagnosing atypical Gitelman's syndrome presenting with normomagnesaemia. Clin Endocrinol (Oxf). 2010; 72:272–6.

9. Colussi G, Bettinelli A, Tedeschi S, De Ferrari ME, Syrén ML, Borsa N, et al. A thiazide test for the diagnosis of renal tubular hypokalemic disorders. Clin J Am Soc Nephrol. 2007; 2:454–60.

10. Fukuyama S, Okudaira S, Yamazato S, Yamazato M, Ohta T. Analysis of renal tubular electrolyte transporter genes in seven patients with hypokalemic metabolic alkalosis. Kidney Int. 2003; 64:808–16.

11. Yoo TH, Lee SH, Yoon K, Baek H, Chung JH, Lee T, et al. Identification of novel mutations in Na-Cl cotransporter gene in a Korean patient with atypical Gitelman's syndrome. Am J Kidney Dis. 2003; 42:E11–6.

12. Lo YF, Nozu K, Iijima K, Morishita T, Huang CC, Yang SS, et al. Recurrent deep intronic mutations in the SLC12A3 gene responsible for Gitelman's syndrome. Clin J Am Soc Nephrol. 2011; 6:630–9.
13. Favero M, Calò LA, Schiavon F, Punzi L. Miscellaneous non-inflammatory musculoskeletal conditions. Bartter's and Gitelman's diseases. Best Pract Res Clin Rheumatol. 2011; 25:637–48.
14. Colussi G, Rombolà G, De Ferrari ME, Macaluso M, Minetti L. Correction of hypokalemia with antialdosterone therapy in Gitelman's syndrome. Am J Nephrol. 1994; 14:127–35.
Go to : 
 | Figure 1.A 12-lead electrocardiogram (ECG) from a 27-year-old woman with hypokalemia. ECG demonstrates flatten T-waves, depressed ST-segment changes, and prominent U waves. |
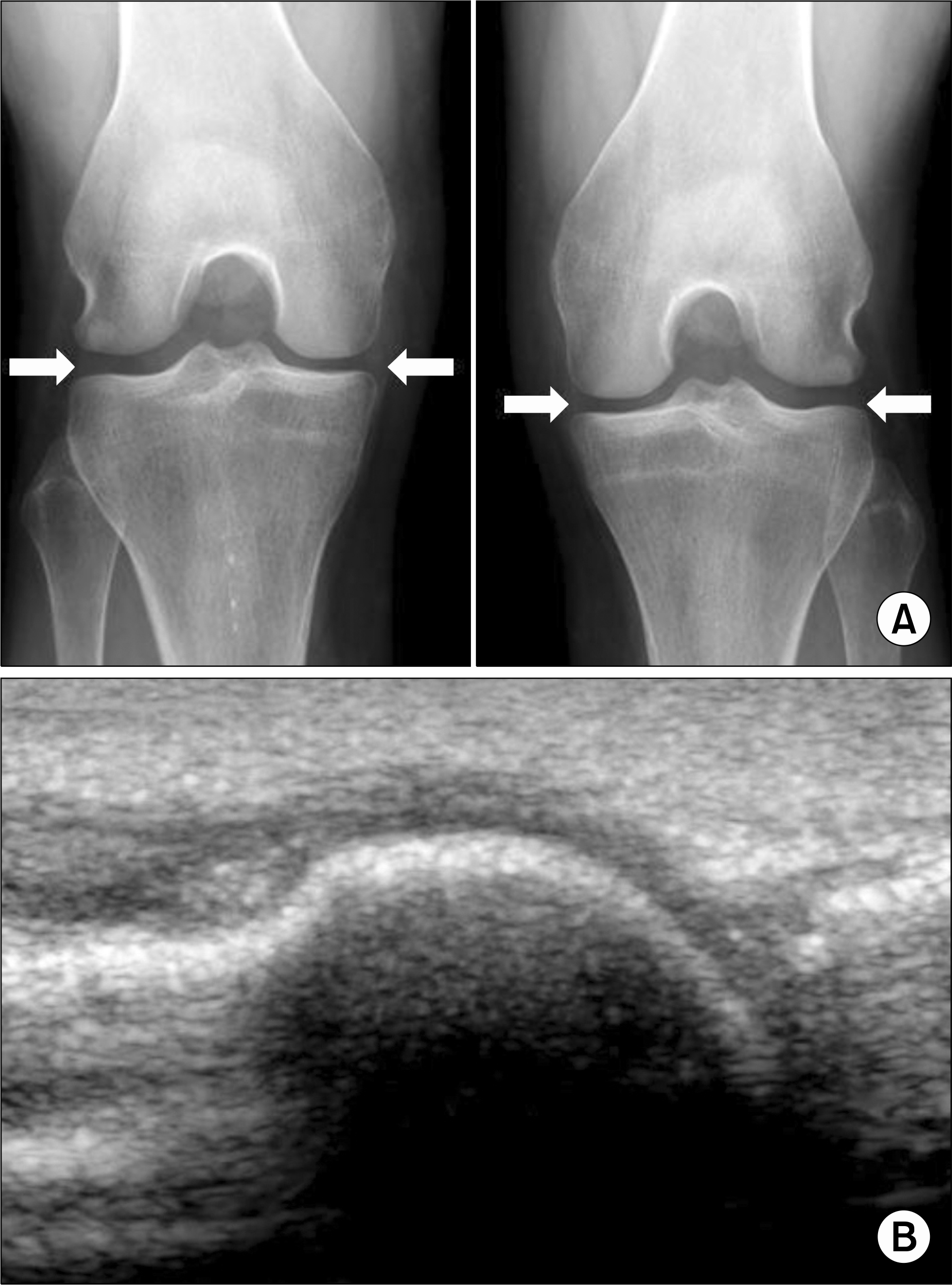 | Figure 2.Chondrocalcinosis in radiographies of the patient's knees (A). Arrows show amorphous radiopaque densities along both medial and lateral menisci. Ultrasonographic examination of right 1st metatarsophalangeal joint (B) showed no evidence of crystal deposition like double contour sign. |
Table 1.
Biochemical data of patient
| Parameter | At first admission (April 2014) | At latest admission (April 2015) | Reference range |
|---|---|---|---|
| Serum chemistry | |||
| Sodium (mEq/L) | 136 | 138 | 135∼145 |
| Potassium (mEq/L) | 2.1 | 2.4 | 3.5∼5.5 |
| Chloride (mEq/L) | 82 | 87 | 96∼110 |
| Magnesium (mg/dL) | 1.9 | 2.4 | 1.5∼2.5 |
| Calcium (mg/dL) | 9.6 | 9.4 | 8.4∼10.4 |
| Phosphate (mg/dL) | 3.0 | 4.5 | 2.5∼4.5 |
| Uric acid (mg/dL) | 9.6 | 9.3 | 2.2∼8 |
| Osmolality (mOsm/kg) | 285 | 290 | 280∼300 |
| BUN (mg/dL) | 37.6 | 24.2 | 7∼20 |
| Creatinine (mg/dL) | 1.22 | 1.08 | 0.6∼1.4 |
| eGFR | 56 | 70 | |
| AST (U/L) | 23 | 25 | 5∼40 |
| ALT (U/L) | 22 | 20 | 5∼45 |
| ALP (U/L) | 81 | 84 | 30∼110 |
| Ferritin (ng/mL) | 101.3 | - | 13∼150 |
| Urine chemistry | (24 h urine) | (Spot urine) | |
| Sodium (mEq/d) | 146.0 | 179 | 40∼220 (25∼250)* |
| Potassium (mEq/d) | 37.6 | 84.7 | 25∼120 (12∼129)* |
| Chloride (mEq/d) | 45.1 | 59 | 110∼250 (0∼300)* |
| Magnesium (mg/d) | 77.1 | - | 7.3∼12.2 |
| Calcium (mg/d) | 13.9 | - | 100∼300 |
| Osmolality (mOsm/kg) | 315 | 622 | 400∼800 |
| Urine pH | 8.5 | 8.5 | 5.0∼8.0 |
| Arterial blood gas analysis | |||
| pH | 7.56 | - | 7.35∼7.45 |
| PaCO2 (mmHg) | 56.6 | - | 32∼45 |
| PaO2 (mmHg) | 85.6 | - | 83∼108 |
| − (mmol/L) HCO3 | 49.2 | - | 21∼28 |
| Base excess (mmol/L) | 23.9 | - | −2∼2 |
| Endocrine test | |||
| Plasma renin activity (ng/mL/h) | 34.78 | - | 0.15∼2.33 |
| Serum aldosterone level (ng/dL) | 11.0 | - | 1.3∼14.5 |
| TSH (μ IU/mL) | 1.33 | 0.27∼4.2 | |
| Free T4 (ng/dL) | 1.57 | 0.93∼1.7 | |
| PTH-intact (pg/mL) | 86.6 | 15∼65 |
Table 2.
Thiazide and furosemide loading test*
Sodium and chloride clearances were markedly increased after furosemide loading, but not affected by thiazide loading. FEa: Solute fractional clearance (%)=[(Ua×Pcr)/(Pa×Ucr)]×100.Δ FE: The difference between maximal excretion at any time after diuretics (thiazide or furosemide) administration and FE (base): ΔFE=FE (max)-FE (base).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download