Abstract
Objective
To examine the demographic profile and treatment patterns in patients with rheumatoid arthritis (RA) prescribed low-dose modified-release prednisone (LODOTRAⓇ) on a named patient basis in Singapore and to evaluate safety and clinical outcome of the treatment.
Methods
Medical records of adult patients with RA who had inadequate responses to prior RA treatment and were prescribed low-dose modified-release prednisone between January and December 2012 at a specialist clinic were reviewed retrospectively. Demographics, treatment information, relevant laboratory evaluations, and disease condition, prior to and after the start of treatment, were collected.
Results
Thirty-eight patients were enrolled. The mean age was 52.8 years and median disease duration was 1.3 years (0.04 to 8.2 years). Patients received a mean daily dose of 5.0±1.0 mg of modi-fied-release prednisone for a median period of 4.4 months (0.2 to 11.8 months). Before treatment, the majority of patients received disease-modifying anti-rheumatic drugs (78.9%), glucocorticoids (71.0%), and non-steroidal anti-inflammatory drugs (NSAIDs) (68.4%). After the start of treatment, prescription of NSAIDs declined from 68.4% to 28.9%. Similar laboratory findings were observed before and after treatment. The median C-reactive protein level decreased substantially from 9.8 mg/L (0.2 to 77.7 mg/L) to 3.9 mg/L (0.4 to 27.6 mg/L). High proportions of patients reported improvement or recovery from morning stiffness (94.7%) or joint pain (70.0 to 100.0%) after treatment. The median number of painful joints decreased from 4 (1 to 8) to 0 (0 to 4) after treatment.
Go to : 
REFERENCES
1. Silman AJ, Hochberg MC. Epidemiology of the rheumatic diseases. 2nd ed.New York: Oxford University Press;2001. p. 31–71.
2. World Health Organization. Chronic diseases and health promotion [Internet]. Geneva: World Health Organization. [cited 2014 Jan 1]. Available from:. http://www.who.int/chp/topics/rheumatic/en/.
3. Haus E, Sackett-Lundeen L, Smolensky MH. Rheumatoid arthritis: circadian rhythms in disease activity, signs and symptoms, and rationale for chronotherapy with corticosteroids and other medications. Bull NYU Hosp Jt Dis. 2012; 70(Suppl 1):3–10.
4. Straub RH, Cutolo M. Circadian rhythms in rheumatoid arthritis: implications for pathophysiology and therapeutic management. Arthritis Rheum. 2007; 56:399–408.

5. Perry MG, Kirwan JR, Jessop DS, Hunt LP. Overnight variations in cortisol, interleukin 6, tumour necrosis factor alpha and other cytokines in people with rheumatoid arthritis. Ann Rheum Dis. 2009; 68:63–8.
6. Straub RH, Paimela L, Peltomaa R, Schölmerich J, Leirisalo-Repo M. Inadequately low serum levels of steroid hormones in relation to interleukin-6 and tumor necrosis factor in untreated patients with early rheumatoid arthritis and reactive arthritis. Arthritis Rheum. 2002; 46:654–62.

7. da Silva JA, Phillips S, Buttgereit F. Impact of impaired morning function on the lives and well-being of patients with rheumatoid arthritis. Scand J Rheumatol Suppl. 2011; 125:6–11.

8. Franke LC, Ament AJ, van de Laar MA, Boonen A, Severens JL. Cost-of-illness of rheumatoid arthritis and ankylosing spondylitis. Clin Exp Rheumatol. 2009; 27:S118–23.
9. Kwong FK, Sue MA, Klaustermeyer WB. Corticosteroid complications in respiratory disease. Ann Allergy. 1987; 58:326–30.
11. Arvidson NG, Gudbjörnsson B, Larsson A, Hällgren R. The timing of glucocorticoid administration in rheumatoid arthritis. Ann Rheum Dis. 1997; 56:27–31.

12. Buttgereit F, Doering G, Schaeffler A, Witte S, Sierakowski S, Gromnica-Ihle E, et al. Efficacy of modified-release versus standard prednisone to reduce duration of morning stiffness of the joints in rheumatoid arthritis (CAPRA-1): a dou-ble-blind, randomised controlled trial. Lancet. 2008; 371:205–14.

13. Nichols T, Nugent CA, Tyler FH. Diurnal variation in suppression of adrenal function by glucocorticoids. J Clin Endocrinol Metab. 1965; 25:343–9.

14. Cutolo M. Chronobiology and the treatment of rheumatoid arthritis. Curr Opin Rheumatol. 2012; 24:312–8.

15. Clarke L, Kirwan J. Efficacy, safety and mechanism of action of modified-release prednisone in rheumatoid arthritis. Ther Adv Musculoskelet Dis. 2012; 4:159–66.

16. Buttgereit F, Mehta D, Kirwan J, Szechinski J, Boers M, Alten RE, et al. Low-dose prednisone chronotherapy for rheumatoid arthritis: a randomised clinical trial (CAPRA-2). Ann Rheum Dis. 2013; 72:204–10.

17. Buttgereit F, Doering G, Schaeffler A, Witte S, Sierakowski S, Gromnica-Ihle E, et al. Targeting pathophysiological rhythms: prednisone chronotherapy shows sustained efficacy in rheumatoid arthritis. Ann Rheum Dis. 2010; 69:1275–80.

18. Alten R, Döring G, Cutolo M, Gromnica-Ihle E, Witte S, Straub R, et al. Hypothalamus-pituitary-adrenal axis function in patients with rheumatoid arthritis treated with nighttime-release prednisone. J Rheumatol. 2010; 37:2025–31.

19. Cutolo M, Iaccarino L, Doria A, Govoni M, Sulli A, Marcassa C. Efficacy of the switch to modified-release prednisone in rheumatoid arthritis patients treated with standard glucocorticoids. Clin Exp Rheumatol. 2013; 31:498–505.
20. American College of Rheumatology Subcommittee on Rheumatoid Arthritis Guidelines. Guidelines for the management of rheumatoid arthritis: 2002 update. Arthritis Rheum. 2002; 46:328–46.
21. Mota LM, Cruz BA, Brenol CV, Pereira IA, Rezende-Fronza LS, Bertolo MB, et al. Sociedade Brasileira de Reumatologia. Guidelines for the drug treatment of rheumatoid arthritis. Rev Bras Reumatol. 2013; 53:158–83.
22. Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012; 64:625–39.

23. Meyer T, Kuhr M, Peissker F, Kostev K. Influence of treatment with modified-release prednisone (LodotraⓇ) on the use of concomitant drugs in patients with active rheumatoid arthritis. Abstracts zum 40. Kongress der Deutschen Gesellschaft für Rheumatologie (DGRh). Z Rheumatol. 2012; 71:1–147.
Go to : 
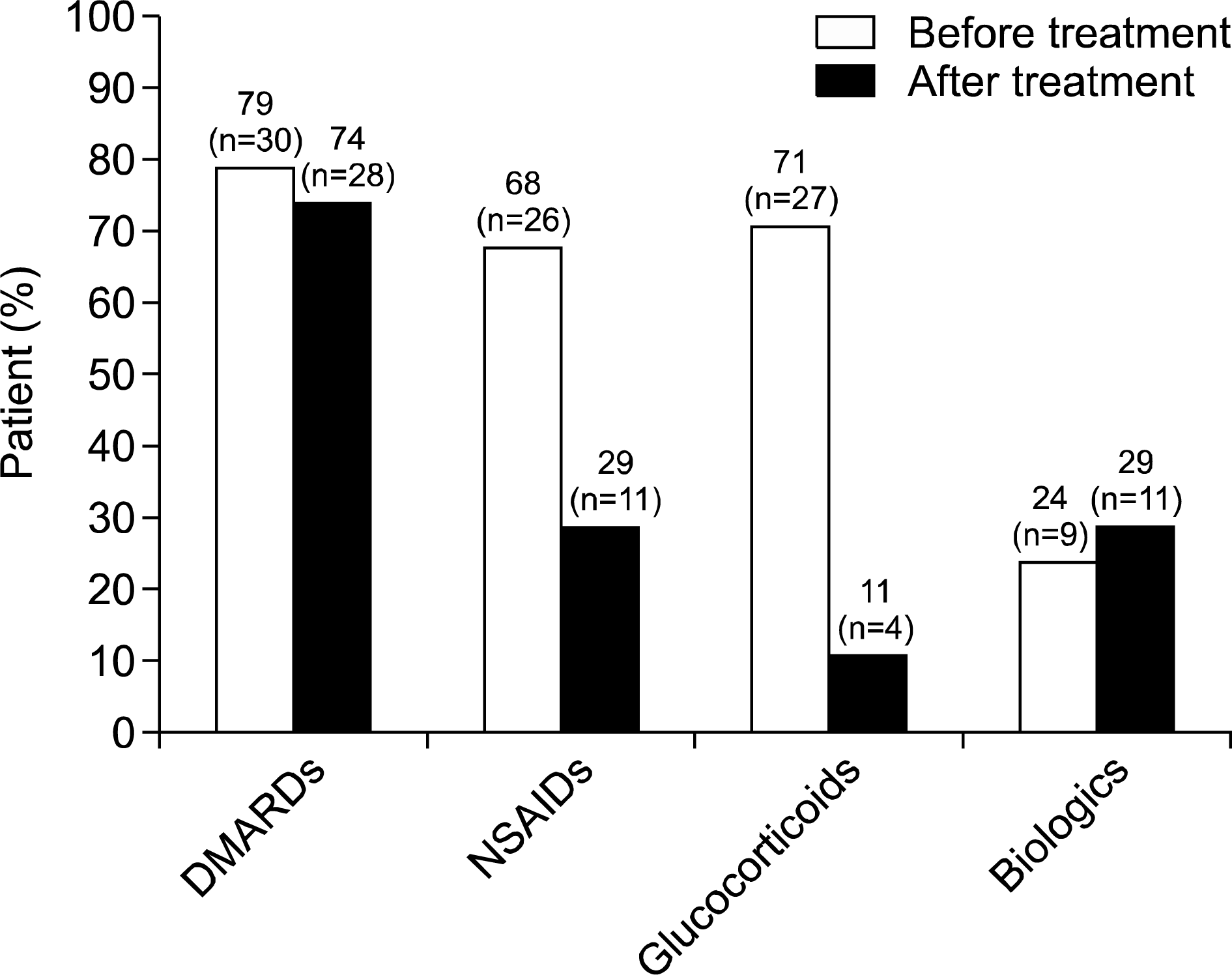 | Figure 1.Prescription of maintenance medication among patients with rheumatoid arthritis before and after the start of low-dose modified-release prednisone chronotherapy. DMARDs: disease modifying anti-rheumatic drugs, NSAIDs: nonsteroidal anti-inflammatory drugs. |
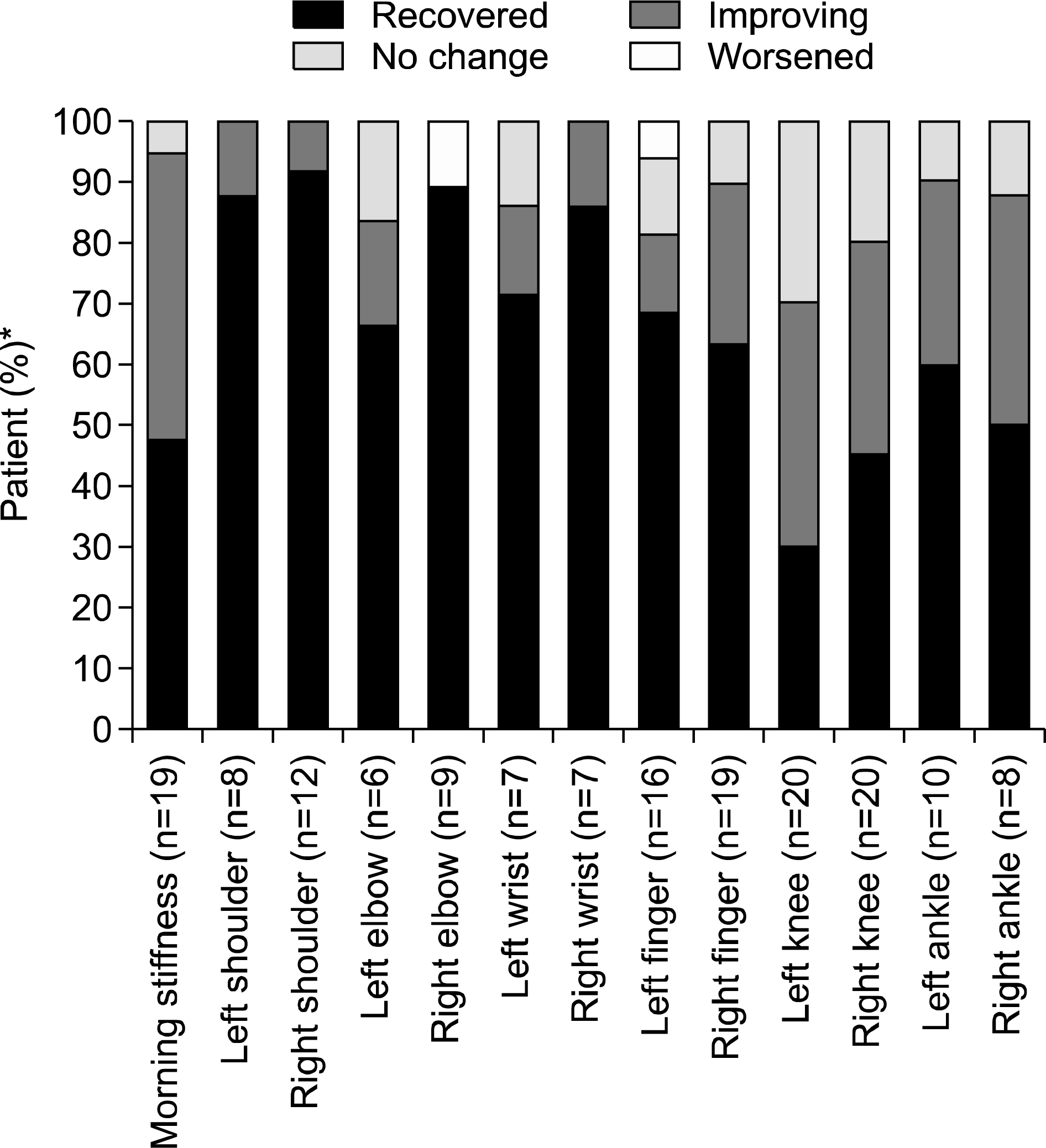 | Figure 2.Course of disease condition after the start of low-dose modified-release prednisone chronotherapy compared to baseline. *Among patients with morning stiffness or pain at baseline. |
Table 1.
Demographics and baseline clinical characteristics (n=38)
| Characteristic | |
|---|---|
| Age (yr) | 52.8±12.8 |
| Female | 35 (92.1) |
| Race | |
| Chinese | 22 (57.9) |
| Indian | 13 (34.2) |
| Malay | 3 (7.9) |
| Disease duration since diagnosis (yr) | 1.3 (0.04∼8.2) |
| Morning stiffness | 19 (50.0) |
| Joint pain | 38 (100.0) |
| Total number of painful joints | 4 (1∼8) |
| Shoulder pain | 15 (39.5)* |
| Left | 8 (21.1) |
| Right | 12 (31.6) |
| Elbow pain | 11 (28.9)* |
| Left | 6 (15.8) |
| Right | 9 (23.7) |
| Wrist pain | 12 (31.6)* |
| Left | 7 (18.4) |
| Right | 7 (18.4) |
| Finger pain | 24 (63.2)* |
| Left | 16 (42.1) |
| Right | 19 (50.0) |
| Knee pain | 27 (71.1)* |
| Left | 20 (52.6) |
| Right | 20 (52.6) |
| Ankle pain | 15 (39.5)* |
| Left | 10 (26.3) |
| Right | 8 (21.1) |
| Other sites | 12 (31.6) |
Table 2.
Maintenance medications prescribed
Table 3.
Flare medications prescribed
Table 4.
Laboratory test findings before and after the start of low-dose modified-release prednisone chronotherapy
| Laboratory finding | Before treatment | After start of treatment |
|---|---|---|
| Fasting glucose level (mg/dL) | 92.5 (70.0∼216.0) | 99.0 (67.0∼156.0) |
| Abnormal* | 4 | 4 |
| Normal | 24 | 12 |
| Unknown | 10 | 22 |
| Triglyceride level (mg/dL) | 100.0 (24.0∼314.0) | 88.0 (34.2∼225.0) |
| Abnormal* | Not determined | 2 |
| Normal | Not determined | 7 |
| Unknown | 11 | 29 |
| Total cholesterol level (mg/dL) | 196.0 (126.0∼303.0) | 218.0 (108.5∼314.0) |
| Abnormal* | Not determined | 2 |
| Normal | Not determined | 7 |
| Unknown | 11 | 29 |
| ALT (U/L) | 16.0 (8.0∼123.0) | 19 (9.0∼87.0) |
| Abnormal* | 1 | 0 |
| Normal | 27 | 16 |
| Unknown | 10 | 29 |
| AST (U/L) | 24.5 (14.0∼66.0) | 22.0 (14.0∼75.0) |
| Abnormal* | 0 | 0 |
| Normal | 28 | 16 |
| Unknown | 10 | 29 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download