Abstract
Mucosal-associated invariant T (MAIT) cells are evolutionarily conserved T cells that are restricted by the non-classical major histocompatibility complex class-1b molecule MR1. MAIT cells recognize riboflavin (vitamin B2) derivatives in a MR1-depen-dent manner. Following antigen recongnition, MAIT cells rapidly produce Th1/Th17 cytokines, such as interferon-γ and inter-leukin-17, in an innate-like manner. MAIT cells maintain an activated phenotype throughout the course of an infection, secrete inflammatory cytokines, and have the potential to directly kill infected cells, thus, playing an important role in controlling the host response. In this review, we discuss current knowledge regarding the role of MAIT cells in infectious diseases, cancers, and autoimmune diseases.
Go to : 
REFERENCES
1. Salio M, Silk JD, Jones EY, Cerundolo V. Biology of CD1− and MR1-restricted T cells. Annu Rev Immunol. 2014; 32:323–66.

2. Howson LJ, Salio M, Cerundolo V. MR1-restricted muco-sal-associated invariant T cells and their activation during infectious diseases. Front Immunol. 2015; 6:303.

3. Porcelli S, Yockey CE, Brenner MB, Balk SP. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4− 8− alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. J Exp Med. 1993; 178:1–16.
4. Tilloy F, Treiner E, Park SH, Garcia C, Lemonnier F, de la Salle H, et al. An invariant T cell receptor alpha chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted alpha/beta T cell subpopulation in mammals. J Exp Med. 1999; 189:1907–21.
5. Martin E, Treiner E, Duban L, Guerri L, Laude H, Toly C, et al. Stepwise development of MAIT cells in mouse and human. PLoS Biol. 2009; 7:e54.

6. Reantragoon R, Corbett AJ, Sakala IG, Gherardin NA, Furness JB, Chen Z, et al. Antigen-loaded MR1 tetramers define T cell receptor heterogeneity in mucosal-associated invariant T cells. J Exp Med. 2013; 210:2305–20.

7. Le Bourhis L, Martin E, Péguillet I, Guihot A, Froux N, Coré M, et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol. 2010; 11:701–8.

8. Savage AK, Constantinides MG, Han J, Picard D, Martin E, Li B, et al. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008; 29:391–403.

9. Sharma PK, Wong EB, Napier RJ, Bishai WR, Ndung'u T, Kasprowicz VO, et al. High expression of CD26 accurately identifies human bacteria-reactive MR1-restricted MAIT cells. Immunology. 2015; 145:443–53.

10. Dusseaux M, Martin E, Serriari N, Péguillet I, Premel V, Louis D, et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood. 2011; 117:1250–9.

12. Beckman EM, Porcelli SA, Morita CT, Behar SM, Furlong ST, Brenner MB. Recognition of a lipid antigen by CD1-re-stricted alpha beta+ T cells. Nature. 1994; 372:691–4.
13. Corbett AJ, Eckle SB, Birkinshaw RW, Liu L, Patel O, Mahony J, et al. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature. 2014; 509:361–5.

14. Kjer-Nielsen L, Patel O, Corbett AJ, Le Nours J, Meehan B, Liu L, et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012; 491:717–23.

15. Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, et al. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003; 422:164–9.

16. Gold MC, Cerri S, Smyk-Pearson S, Cansler ME, Vogt TM, Delepine J, et al. Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol. 2010; 8:e1000407.

17. Mattner J, Debord KL, Ismail N, Goff RD, Cantu C 3rd, Zhou D, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005; 434:525–9.

18. Le Bourhis L, Mburu YK, Lantz O. MAIT cells, surveyors of a new class of antigen: development and functions. Curr Opin Immunol. 2013; 25:174–80.

19. Huang S, Martin E, Kim S, Yu L, Soudais C, Fremont DH, et al. MR1 antigen presentation to mucosal-associated invariant T cells was highly conserved in evolution. Proc Natl Acad Sci U S A. 2009; 106:8290–5.

20. Hashimoto K, Hirai M, Kurosawa Y. A gene outside the human MHC related to classical HLA class I genes. Science. 1995; 269:693–5.

21. Riegert P, Wanner V, Bahram S. Genomics, isoforms, expression, and phylogeny of the MHC class I-related MR1 gene. J Immunol. 1998; 161:4066–77.
22. Huang S, Gilfillan S, Kim S, Thompson B, Wang X, Sant AJ, et al. MR1 uses an endocytic pathway to activate mucosal-associated invariant T cells. J Exp Med. 2008; 205:1201–11.

23. Chua WJ, Kim S, Myers N, Huang S, Yu L, Fremont DH, et al. Endogenous MHC-related protein 1 is transiently expressed on the plasma membrane in a conformation that activates mucosal-associated invariant T cells. J Immunol. 2011; 186:4744–50.

24. Gold MC, McLaren JE, Reistetter JA, Smyk-Pearson S, Ladell K, Swarbrick GM, et al. MR1-restricted MAIT cells display ligand discrimination and pathogen selectivity through distinct T cell receptor usage. J Exp Med. 2014; 211:1601–10.

25. Cho YN, Kee SJ, Kim TJ, Jin HM, Kim MJ, Jung HJ, et al. Mucosal-associated invariant T cell deficiency in systemic lupus erythematosus. J Immunol. 2014; 193:3891–901.

26. Reantragoon R, Kjer-Nielsen L, Patel O, Chen Z, Illing PT, Bhati M, et al. Structural insight into MR1-mediated recognition of the mucosal associated invariant T cell receptor. J Exp Med. 2012; 209:761–74.

27. Lepore M, Kalinichenko A, Colone A, Paleja B, Singhal A, Tschumi A, et al. Parallel T-cell cloning and deep sequencing of human MAIT cells reveal stable oligoclonal TCRβ repertoire. Nat Commun. 2014; 5:3866.

28. Napier RJ, Adams EJ, Gold MC, Lewinsohn DM. The role of mucosal associated invariant T cells in antimicrobial immunity. Front Immunol. 2015; 6:344.

29. Leeansyah E, Loh L, Nixon DF, Sandberg JK. Acquisition of innate-like microbial reactivity in mucosal tissues during human fetal MAIT-cell development. Nat Commun. 2014; 5:3143.

30. Turtle CJ, Delrow J, Joslyn RC, Swanson HM, Basom R, Tabellini L, et al. Innate signals overcome acquired TCR signaling pathway regulation and govern the fate of human CD161(hi) CD8α+ semi-invariant T cells. Blood. 2011; 118:2752–62.
31. Ussher JE, Bilton M, Attwod E, Shadwell J, Richardson R, de Lara C, et al. CD161++ CD8+ T cells, including the MAIT cell subset, are specifically activated by IL-12+ IL-18 in a TCR-independent manner. Eur J Immunol. 2014; 44:195–203.
32. Tang XZ, Jo J, Tan AT, Sandalova E, Chia A, Tan KC, et al. IL-7 licenses activation of human liver intrasinusoidal mucosal-associated invariant T cells. J Immunol. 2013; 190:3142–52.

33. Le Bourhis L, Dusseaux M, Bohineust A, Bessoles S, Martin E, Premel V, et al. MAIT cells detect and efficiently lyse bacterially-infected epithelial cells. PLoS Pathog. 2013; 9:e1003681.

34. Kurioka A, Ussher JE, Cosgrove C, Clough C, Fergusson JR, Smith K, et al. MAIT cells are licensed through granzyme exchange to kill bacterially sensitized targets. Mucosal Immunol. 2015; 8:429–40.

35. Pinkoski MJ, Hobman M, Heibein JA, Tomaselli K, Li F, Seth P, et al. Entry and trafficking of granzyme B in target cells during granzyme B-perforin-mediated apoptosis. Blood. 1998; 92:1044–54.

36. Harari A, Bellutti Enders F, Cellerai C, Bart PA, Pantaleo G. Distinct profiles of cytotoxic granules in memory CD8 T cells correlate with function, differentiation stage, and antigen exposure. J Virol. 2009; 83:2862–71.

37. Billerbeck E, Kang YH, Walker L, Lockstone H, Grafmueller S, Fleming V, et al. Analysis of CD161 expression on human CD8+ T cells defines a distinct functional subset with tissue-homing properties. Proc Natl Acad Sci USA. 2010; 107:3006–11.

38. Annibali V, Ristori G, Angelini DF, Serafini B, Mechelli R, Cannoni S, et al. CD161(high)CD8+ T cells bear pathogenetic potential in multiple sclerosis. Brain. 2011; 134:542–54.
39. Teunissen MB, Yeremenko NG, Baeten DL, Chielie S, Spuls PI, de Rie MA, et al. The IL-17A-producing CD8+ T-cell population in psoriatic lesional skin comprises muco-sa-associated invariant T cells and conventional T cells. J Invest Dermatol. 2014; 134:2898–907.
40. Lee OJ, Cho YN, Kee SJ, Kim MJ, Jin HM, Lee SJ, et al. Circulating mucosal-associated invariant T cell levels and their cytokine levels in healthy adults. Exp Gerontol. 2014; 49:47–54.

41. Ussher JE, Klenerman P, Willberg CB. Mucosal-associated invariant T-cells: new players in anti-bacterial immunity. Front Immunol. 2014; 5:450.

42. Wong EB, Akilimali NA, Govender P, Sullivan ZA, Cosgrove C, Pillay M, et al. Low levels of peripheral CD161++ CD8+ mucosal associated invariant T (MAIT) cells are found in HIV and HIV/TB co-infection. PLoS One. 2013; 8:e83474.
43. Kwon YS, Cho YN, Kim MJ, Jin HM, Jung HJ, Kang JH, et al. Mucosal-associated invariant T cells are numerically and functionally deficient in patients with mycobacterial infection and reflect disease activity. Tuberculosis (Edinb). 2015; 95:267–74.

44. Jiang J, Wang X, An H, Yang B, Cao Z, Liu Y, et al. Mucosal-associated invariant T-cell function is modulated by programmed death-1 signaling in patients with active tuberculosis. Am J Respir Crit Care Med. 2014; 190:329–39.
45. Kim JC, Jin HM, Cho YN, Kwon YS, Kee SJ, Park YW. Deficiencies of circulating mucosal-associated invariant T cells and natural killer T cells in patients with acute cholecystitis. J Korean Med Sci. 2015; 30:606–11.

46. Grimaldi D, Le Bourhis L, Sauneuf B, Dechartres A, Rousseau C, Ouaaz F, et al. Specific MAIT cell behaviour among innate-like T lymphocytes in critically ill patients with severe infections. Intensive Care Med. 2014; 40:192–201.

47. Leeansyah E, Ganesh A, Quigley MF, Sönnerborg A, Andersson J, Hunt PW, et al. Activation, exhaustion, and persistent decline of the antimicrobial MR1-restricted MAIT-cell population in chronic HIV-1 infection. Blood. 2013; 121:1124–35.

48. Cosgrove C, Ussher JE, Rauch A, Gärtner K, Kurioka A, Hühn MH, et al. Early and nonreversible decrease of CD161++/MAIT cells in HIV infection. Blood. 2013; 121:951–61.

49. Ashare A, Stanford C, Hancock P, Stark D, Lilli K, Birrer E, et al. Chronic liver disease impairs bacterial clearance in a human model of induced bacteremia. Clin Transl Sci. 2009; 2:199–205.

50. Tandon P, Garcia-Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin Liver Dis. 2008; 28:26–42.

51. Greathead L, Metcalf R, Gazzard B, Gotch F, Steel A, Kelleher P. CD8+/CD161++ mucosal-associated invariant T-cell levels in the colon are restored on long-term anti-retroviral therapy and correlate with CD8+ T-cell immune activation. AIDS. 2014; 28:1690–2.
52. Illés Z, Shimamura M, Newcombe J, Oka N, Yamamura T. Accumulation of Valpha7.2-Jalpha33 invariant T cells in human autoimmune inflammatory lesions in the nervous system. Int Immunol. 2004; 16:223–30.
53. Croxford JL, Miyake S, Huang YY, Shimamura M, Yamamura T. Invariant V(alpha)19i T cells regulate autoimmune inflammation. Nat Immunol. 2006; 7:987–94.
54. Miyazaki Y, Miyake S, Chiba A, Lantz O, Yamamura T. Mucosal-associated invariant T cells regulate Th1 response in multiple sclerosis. Int Immunol. 2011; 23:529–35.

55. Serriari NE, Eoche M, Lamotte L, Lion J, Fumery M, Marcelo P, et al. Innate mucosal-associated invariant T (MAIT) cells are activated in inflammatory bowel diseases. Clin Exp Immunol. 2014; 176:266–74.

56. Dunne MR, Elliott L, Hussey S, Mahmud N, Kelly J, Doherty DG, et al. Persistent changes in circulating and intestinal γδ T cell subsets, invariant natural killer T cells and mucosal-associated invariant T cells in children and adults with coeliac disease. PLoS One. 2013; 8:e76008.

57. Chiba A, Tajima R, Tomi C, Miyazaki Y, Yamamura T, Miyake S. Mucosal-associated invariant T cells promote inflammation and exacerbate disease in murine models of arthritis. Arthritis Rheum. 2012; 64:153–61.

58. Peterfalvi A, Gomori E, Magyarlaki T, Pal J, Banati M, Javorhazy A, et al. Invariant Valpha7.2-Jalpha33 TCR is expressed in human kidney and brain tumors indicating infiltration by mucosal-associated invariant T (MAIT) cells. Int Immunol. 2008; 20:1517–25.
Go to : 
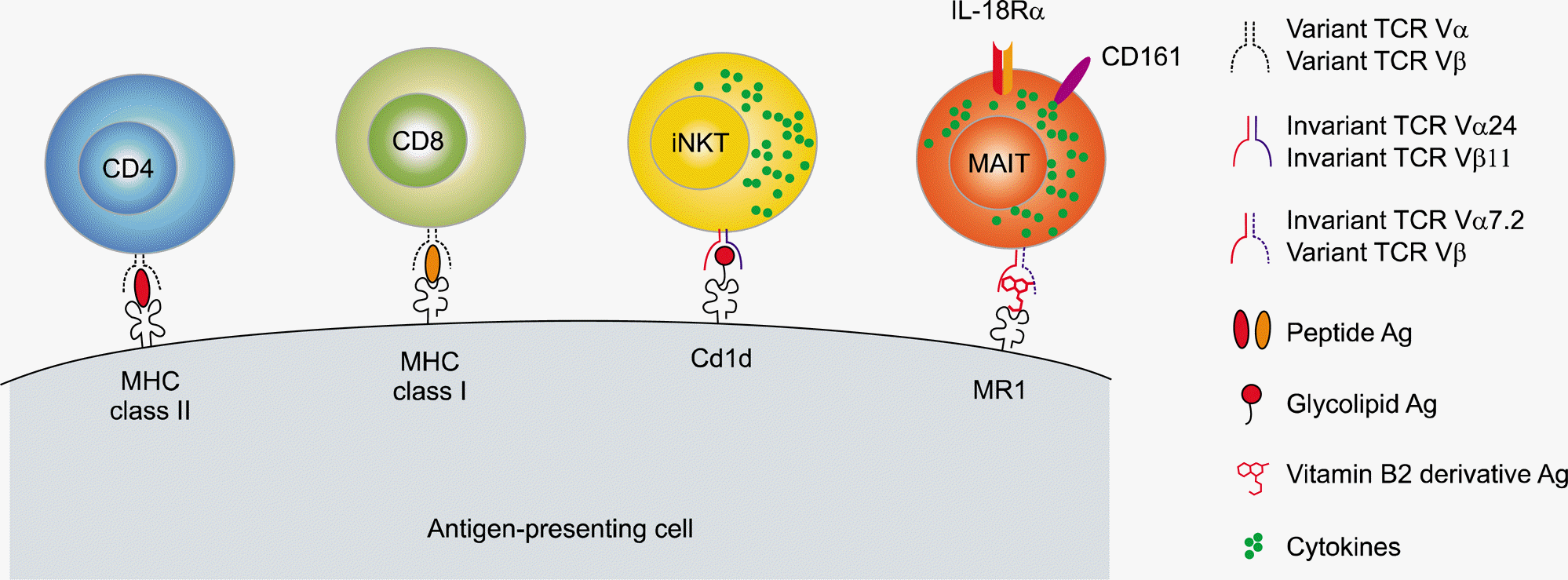 | Figure 1.A schematic of key differences among conventional T cells, invariant natural killer T (iNKT) cells, and mucosal-associated invariant T (MAIT) cells in humans. In contrast to conventional adaptive T cells expressing both diverse T cell receptor (TCR) Vα and Vβ chains, MAIT cells and iNKT cells constitute two major subsets of innate-like T cells with semi-invariant TCR repertoires using TCR Vα7.2-Jα33 chains expressed together with selected TCR β chains and TCR Vα24-Jα33 chains paired with TCR Vβ11 chains, respectively. In addition, MAIT cells are characterized by a high expression of CD161 and interleukin-18Rα (IL-18Rα). MAIT cells and NKT cells recognize vitamin B2 metabolite antigens (Ag) and glycolipid Ag presented by the invariant major histocompatibility complex (MHC)-related 1 (MR1) molecule and MHC class 1-like CD1d molecule, respectively, which are both expressed on Ag-presenting cells. Upon Ag recognition, these innate-like T cells rapidly produce cytokines. |
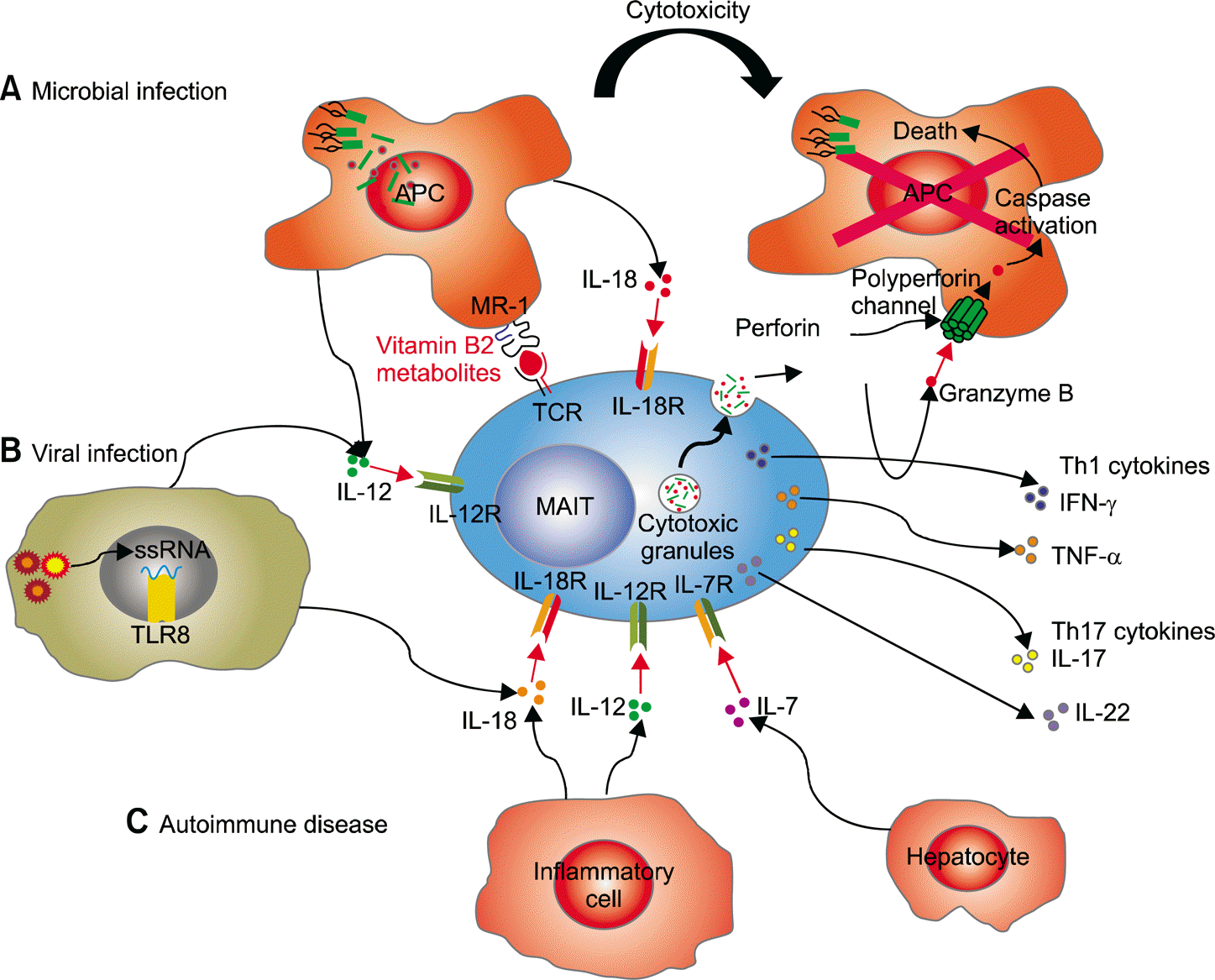 | Figure 2.Mechanisms of mucosal-associated invariant T (MAIT) cell activation in human diseases. (A) Microbial infection. Bacteria or yeast stimulate MAIT cells in an MR1-dependent manner. MAIT cells are activated through Vα7.2-bearing MAIT cell T cell receptor (TCR) recognition of microbial-derived vitamin B metabolite antigens presented by MR1 on infected antigen-presenting cells (APCs). Upon infection, MAIT cells are activated by stimulation with interleukin (IL)-12 and IL-18 produced by APCs in an MR1-independent manner. Furthermore, activation of MAIT cells can be enhanced by other stimuli, such as IL-7 secreted by hep-atocytes in synergy with TCR stimulation. Activated MAIT cells produce Th1 cytokines (interferon [IFN]-γ and tumor necrosis factor [TNF]-α) and Th17 cytokines (IL-17 and IL-22) and directly release perforin and granzyme B, which are cytotoxic effector molecules, to kill infected cells. (B) Viral infection. Viruses stimulate MAIT cells in an MR1-independent manner. Virus-infected cells are activated by detection of their molecular patterns by pattern recognition receptors, such as ssRNA by TLR8, resulting in IL-12 and IL-18 production. (C) Autoimmune disease. MAIT cells are stimulated by IL-12 and IL-18 produced by pathologically activated inflammatory cells present in autoimmune diseases. Figure adapted from an original drawing by Howson et al. (Front Immunol 2015;6:303) [2]. |
Table 1.
Key differences between iNKT cells and MAIT cells




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download