Abstract
Objective
The purpose of this study is to evaluate the outcome of uveitis in ankylosing spondylitis (AS) during tumor necrosis factor (TNF)-inhibiting therapy and to compare the incidence rate of uveitis in infliximab, adalimumab, and etanercept.
Methods
A retrospective evaluation was performed in AS patients who had started TNF-inhibiting therapy from June 2003 to June 2011. The clinical characteristics of patients with documented uveitis were evaluated.
Results
Among 316 patients treated with TNF inhibitor, 26 patients (8%) had experienced uveitis during TNF-inhibiting therapy. Among them, 15 patients were treated with etanercept, eight with adalimumab, and three with infliximab. The overall incidence rate of uveitis flare during therapy with TNF inhibitor was 46 per 1,000 person-years (pys) (95% confidence interval [CI], 32 to 64). The incidence rate did not differ between TNF inhibitors, with 54/1,000 pys (95% CI, 34 to 81) for etanercept, 46/1,000 pys (95% CI, 21 to 87) for adalimumab, and 22/1,000 pys (95% CI, 5 to 64) for infliximab. Fourteen patients experienced a first episode of uveitis. The overall incidence rate of new onset-uveitis after therapy with TNF inhibitor was 19 per 1,000 pys (95% CI, 10 to 31). The incidence rate for etanercept was 24/1,000 pys (95% CI, 12 to 45); adalimumab, 15/1,000 pys (95% CI, 3 to 45); and infliximab, 7/1,000 pys (95% CI, 0 to 40). There was no statistical difference in the incidence of uveitis flare or the cumulative uveitis-free rate among the three TNF inhibitors.
Go to : 
REFERENCES
1. Rosenbaum JT. Acute anterior uveitis and spondyloarthropathies. Rheum Dis Clin North Am. 1992; 18:143–51.

2. Zeboulon N, Dougados M, Gossec L. Prevalence and characteristics of uveitis in the spondyloarthropathies: a systematic literature review. Ann Rheum Dis. 2008; 67:955–9.

3. Coates LC, McGonagle DG, Bennett AN, Emery P, Marzo-Ortega H. Uveitis and tumour necrosis factor blockade in ankylosing spondylitis. Ann Rheum Dis. 2008; 67:729–30.

4. Kakkassery V, Mergler S, Pleyer U. Anti-TNF-alpha treatment: a possible promoter in endogenous uveitis? Observational report on six patients: occurrence of uveitis following etanercept treatment. Curr Eye Res. 2010; 35:751–6.
5. Lim LL, Fraunfelder FW, Rosenbaum JT. Do tumor necrosis factor inhibitors cause uveitis? A registry-based study. Arthritis Rheum. 2007; 56:3248–52.

6. Cobo-Ibáñez T, del Carmen Ordóñez M, Muñoz-Fernández S, Madero-Prado R, Martín-Mola E. Do TNF-blockers reduce or induce uveitis? Rheumatology (Oxford). 2008; 47:731–2.
7. Gaujoux-Viala C, Giampietro C, Gaujoux T, Ea HK, Prati C, Orcel P, et al. Scleritis: a paradoxical effect of etanercept? Etanercept-associated inflammatory eye disease. J Rheumatol. 2012; 39:233–9.

8. van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984; 27:361–8.
9. Rosenbaum JT. Effect of etanercept on iritis in patients with ankylosing spondylitis. Arthritis Rheum. 2004; 50:3736–7.

10. Braun J, Baraliakos X, Listing J, Sieper J. Decreased incidence of anterior uveitis in patients with ankylosing spondylitis treated with the anti-tumor necrosis factor agents infliximab and etanercept. Arthritis Rheum. 2005; 52:2447–51.

11. Guignard S, Gossec L, Salliot C, Ruyssen-Witrand A, Luc M, Duclos M, et al. Efficacy of tumour necrosis factor blockers in reducing uveitis flares in patients with spondylarthropathy: a retrospective study. Ann Rheum Dis. 2006; 65:1631–4.

12. Sieper J, Koenig A, Baumgartner S, Wishneski C, Foehl J, Vlahos B, et al. Analysis of uveitis rates across all etanercept ankylosing spondylitis clinical trials. Ann Rheum Dis. 2010; 69:226–9.

13. Ranganathan P. Pharmacogenomics of tumor necrosis factor antagonists in rheumatoid arthritis. Pharmacogenomics. 2005; 6:481–90.

14. Van den Brande JM, Braat H, van den Brink GR, Versteeg HH, Bauer CA, Hoedemaeker I, et al. Infliximab but not etanercept induces apoptosis in lamina propria T-lymphocytes from patients with Crohn's disease. Gastroenterology. 2003; 124:1774–85.

Go to : 
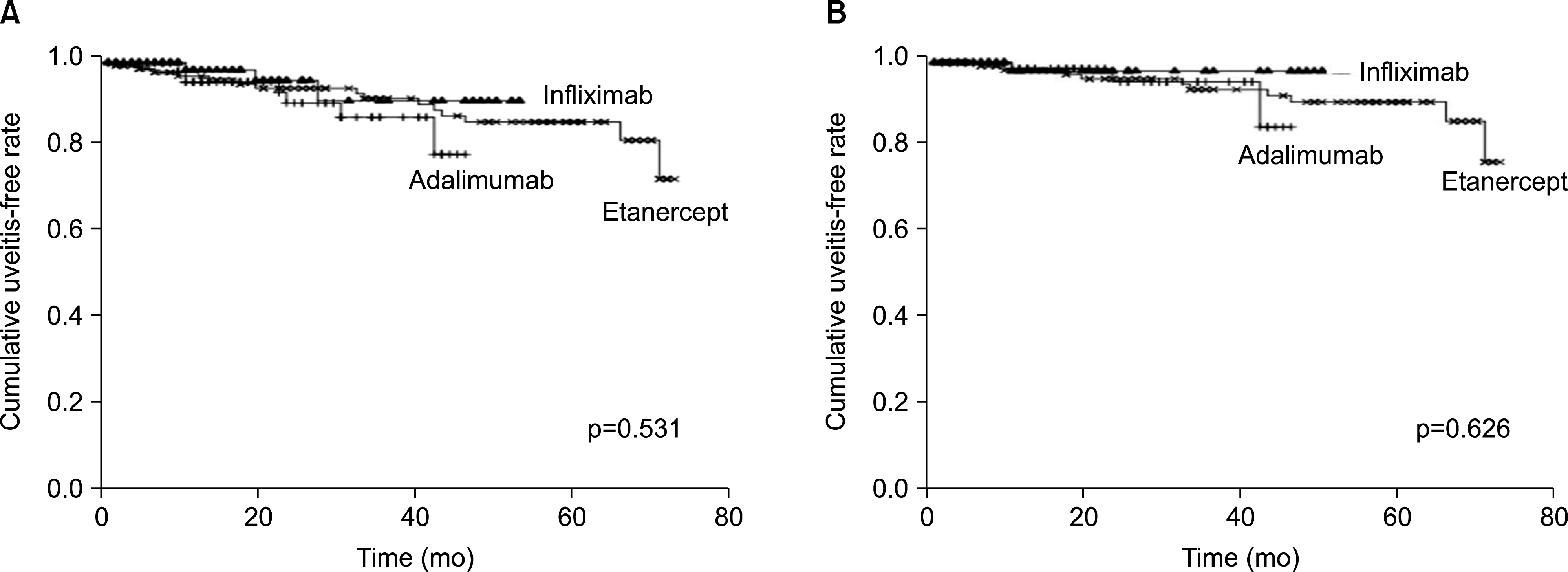 | Figure 1.Cumulative uveitis-free survival rate after tumor necrosis fator (TNF) inhibitors (A) in all patients and (B) in patients who had never experienced uveitis before TNF-inhibiting therapy. |
Table 1.
Comparison of the clinical characteristics of AS patients treated with infliximab, adalimumab, or etanercept
| Characteristic | Total†(n=316) | Infliximab (n=85) | Adalimumab (n=148) | Etanercept (n=142) | p-value |
|---|---|---|---|---|---|
| Age at the start of TNFi (yr) | 36.1±11.8 | 36.4±11.6 | 36.3±11.6 | 36.4±12.4 | 0.987 |
| Male (%) | 81 | 77 | 74 | 83 | 0.189 |
| HLA-B27 positivity (%) | 95 | 95 | 94 | 95 | 0.901 |
| AS disease duration (mo) | 75.7±52.9 | 86.1±54.8 | 58.63±50.1 | 86.8±51.1 | 0.000 |
| Duration of TNFi exposure (mo) | 23.9±19.4 | 19.5±15.0 | 16.1±12.5 | 34.6±22.6 | 0.001 |
| Body mass index (kg/m2) | 24.2±3.9 | 23.9±3.5 | 24.5±4.3 | 24.1±4.0 | 0.598 |
| Previous history of uveitis | 42 (13) | 6 (7) | 21 (14) | 15 (11) | 0.252 |
| Uveitis during TNFi therapy | 26 (8) | 3 (4) | 8 (5) | 15 (11) | 0.000 |
| Uveitis during TNFi therapy (episode) | 34 (11) | 3 (4) | 9 (6) | 22 (15) | 0.004 |
| Incidence rate of uveitis during TNFi therapy (per 1,000 pys) | 46 (32∼64) | 22 (5∼64) | 46 (21∼87) | 54 (34∼81) | 0.257* |
| New onset of uveitis during TNFi therapy | 14 (4) | 1 (1) | 3 (2) | 10 (7) | 0.029 |
| Incidence rate of new onset of uveitis during TNFi therapy (per 1,000 pys) | 19 (10∼31) | 7 (0∼40) | 15 (3∼45) | 24 (12∼45) | 0.357* |
Table 2.
Cox proportional hazard analysis for uveitis of ankylosing spondylitis patients treated with infliximab, adalimumab, or etanercept
| Characteristic | Infliximab (n=85) | Adalimumab (n=148) | Etanercept (n=142) | ||
|---|---|---|---|---|---|
| 95% CI | p | 95% CI | p | ||
| HR developing uveitis during TNFi therapy | Reference | 2.0 (0.5∼7.8) | 0.295 | 1.6 (0.5∼5.8) | 0.461 |
| Adjusted HR developing uveitis during TNFi | Reference | 1.8 (0.4∼7.9) | 0.429 | 1.7 (0.4∼6.5) | 0.443 |
| therapy* | |||||
| HR of new onset of uveitis | Reference | 2.8 (0.3∼28.2) | 0.37 | 2.8 (0.3∼22.7) | 0.336 |
| Adjusted HR of new onset of uveitis* | Reference | 0.9 (0.1∼12.3) | 0.98 | 3.1 (0.3∼27.0) | 0.312 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download