Abstract
Sarcoidosis is a systemic inflammatory granulomatous disease affecting multiple organs, including liver, spleen, heart, eyes, and skin. Liver involvement is reported in 11.5% of cases and many studies have reported on the association between hepatitis C virus infection and sarcoidosis. However, the role of hepatitis B virus (HBV) infection as a trigger for sarcoidosis has never been reported. We describe a case of hepatic sarcoidosis in a patient with chronic hepatitis B infection, with a possible link between the two. It is the first case report of a patient with interferon-α-naïve chronic HBV infection presenting with hepatic sarcoidosis accompanied by portal hypertension and liver cirrhosis.
REFERENCES
1. Blich M, Edoute Y. Clinical manifestations of sarcoid liver disease. J Gastroenterol Hepatol. 2004; 19:732–7.

2. Tan CB, Rashid S, Rajan D, Gebre W, Mustacchia P. Hepatic sarcoidosis presenting as portal hypertension and liver cirrhosis: case report and review of the literature. Case Rep Gastroenterol. 2012; 6:183–9.

3. Rybicki BA, Iannuzzi MC. Epidemiology of sarcoidosis: recent advances and future prospects. Semin Respir Crit Care Med. 2007; 28:22–35.

5. Brjalin V, Salupere R, Tefanova V, Prikk K, Lapidus N, Jõeste E. Sarcoidosis and chronic hepatitis C: a case report. World J Gastroenterol. 2012; 18:5816–20.

6. Baughman RP, Teirstein AS, Judson MA, Rossman MD, Yeager H Jr, Bresnitz EA, et al. Case Control Etiologic Study of Sarcoidosis (ACCESS) research group. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001; 164:1885–9.

7. Cremers J, Drent M, Driessen A, Nieman F, Wijnen P, Baughman R, et al. Liver-test abnormalities in sarcoidosis. Eur J Gastroenterol Hepatol. 2012; 24:17–24.

8. Ramos-Casals M, Mañá J, Nardi N, Brito-Zerón P, Xaubet A, Sánchez-Tapias JM, et al. HISPAMEC Study Group. Sarcoidosis in patients with chronic hepatitis C virus infection: analysis of 68 cases. Medicine (Baltimore). 2005; 84:69–80.
9. Mahévas M, Le Page L, Salle V, Lescure FX, Smail A, Cevallos R, et al. Thrombocytopenia in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2006; 23:229–35.
10. Blum L, Serfaty L, Wattiaux MJ, Picard O, Cabane J, Imbert J. Nodules hypodermiques sarcoïdosiques au cours d'une hépatite virale C traitée par Interféron alpha 2 b. La Revue de Médecine Interne. 1993; 14:1161.

11. Adla M, Downey KK, Ahmad J. Hepatic sarcoidosis associated with pegylated interferon alfa therapy for chronic hepatitis C: case report and review of literature. Dig Dis Sci. 2008; 53:2810–2.
12. Belgodere X, Viraben R, Gorguet B, Allaouchiche B, Lieutaud O, Maestracci D. Guess what! Cutaneous sarcoidosis, Sjögren's syndrome and autoimmune thyroiditis associated with hepatitis C virus infection. Eur J Dermatol. 1999; 9:235–6.
13. Husa P, Klusáková J, Jancíková J, Husová L, Horálek F. Sarcoidosis associated with interferon-alpha therapy for chronic hepatitis B. Eur J Intern Med. 2002; 13:129–31.

14. Bertoletti A, D'Elios MM, Boni C, De Carli M, Zignego AL, Durazzo M, et al. Different cytokine profiles of intra-phepatic T cells in chronic hepatitis B and hepatitis C virus infections. Gastroenterology. 1997; 112:193–9.

15. Moller DR. Cells and cytokines involved in the pathogenesis of sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 1999; 16:24–31.
Figure 1.
Abdomen sonogram (A) and abdomen computed tomography (CT) scan (B). Sonogram (A) reveals coarse parenchymal echogenicity of the liver. CT scan (B) shows heterogeneous enhancement in the enlarged liver and the spleen.
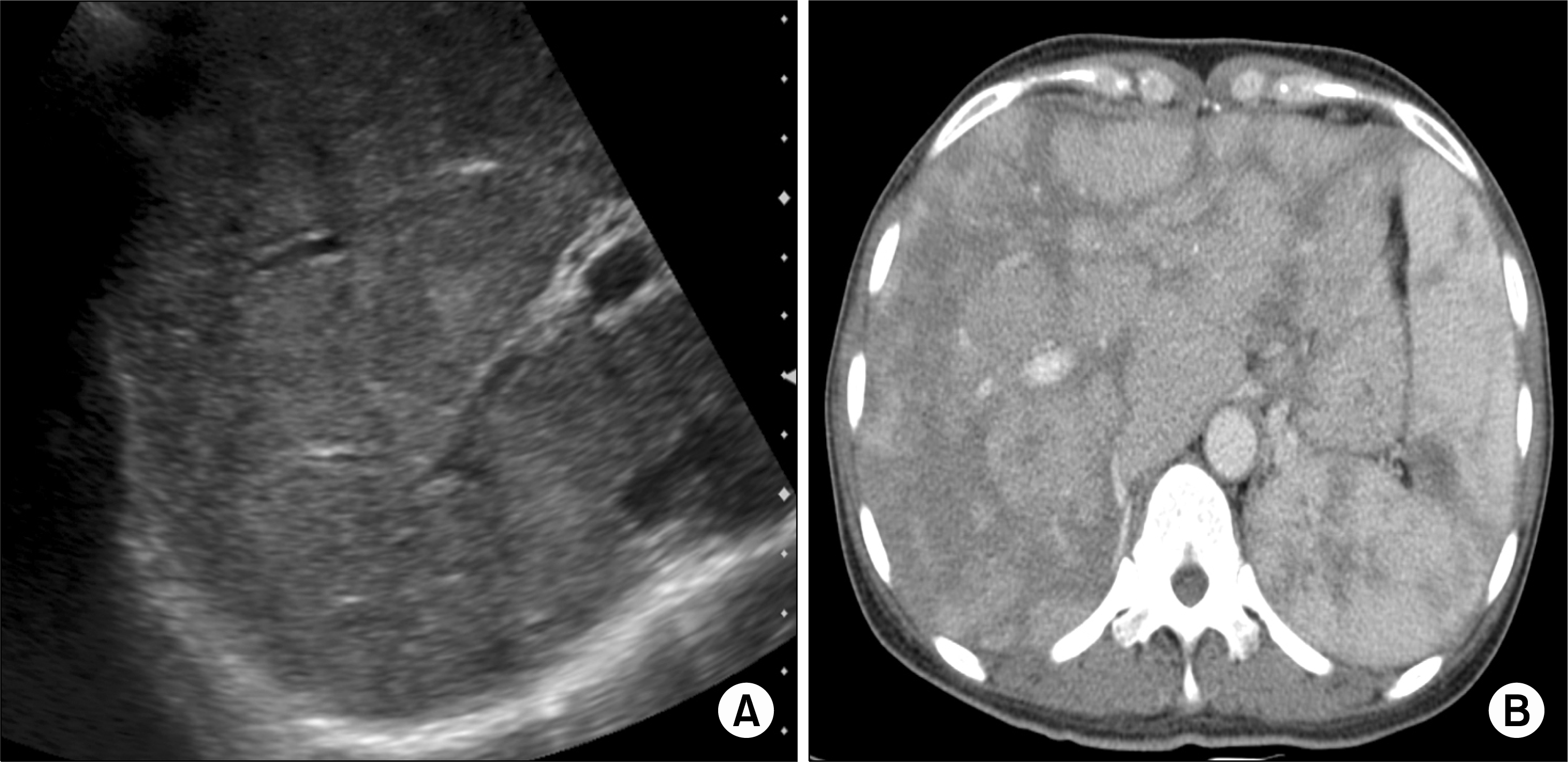
Figure 2.
Liver biopsy showing the non-caseating granuloma (arrows) (A) and asteroid body (B). Special staining for acid fast bacilli (AFB) (C) and periodic acid Schiff (PAS) (D) were negative (A: H&E, ×100; B: H&E, ×400; C: AFB, ×400; D: PAS, ×400).
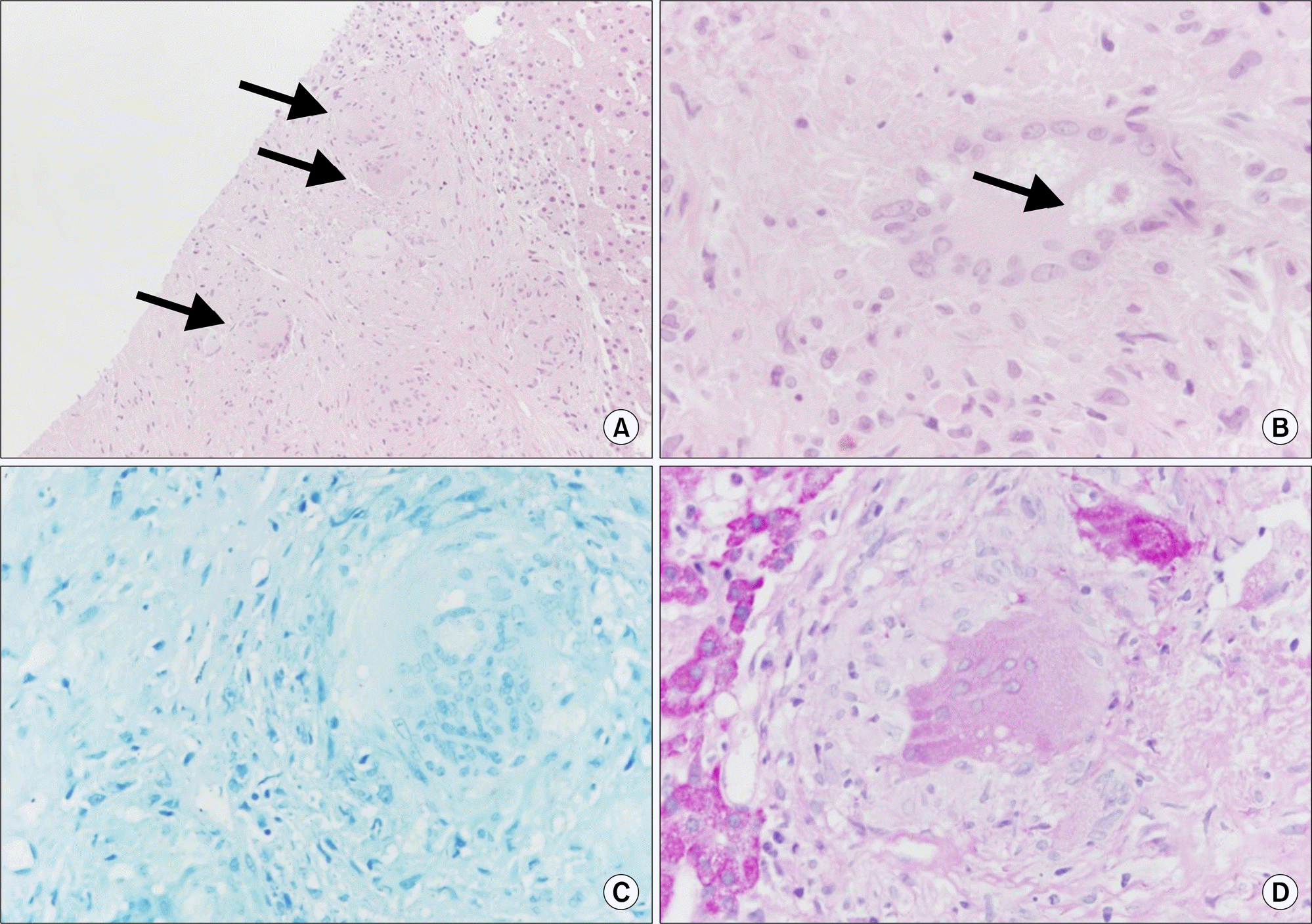
Figure 3.
Positron emission tomography scan with multiple uptakes in the liver, spleen, lymph nodes, and bones.
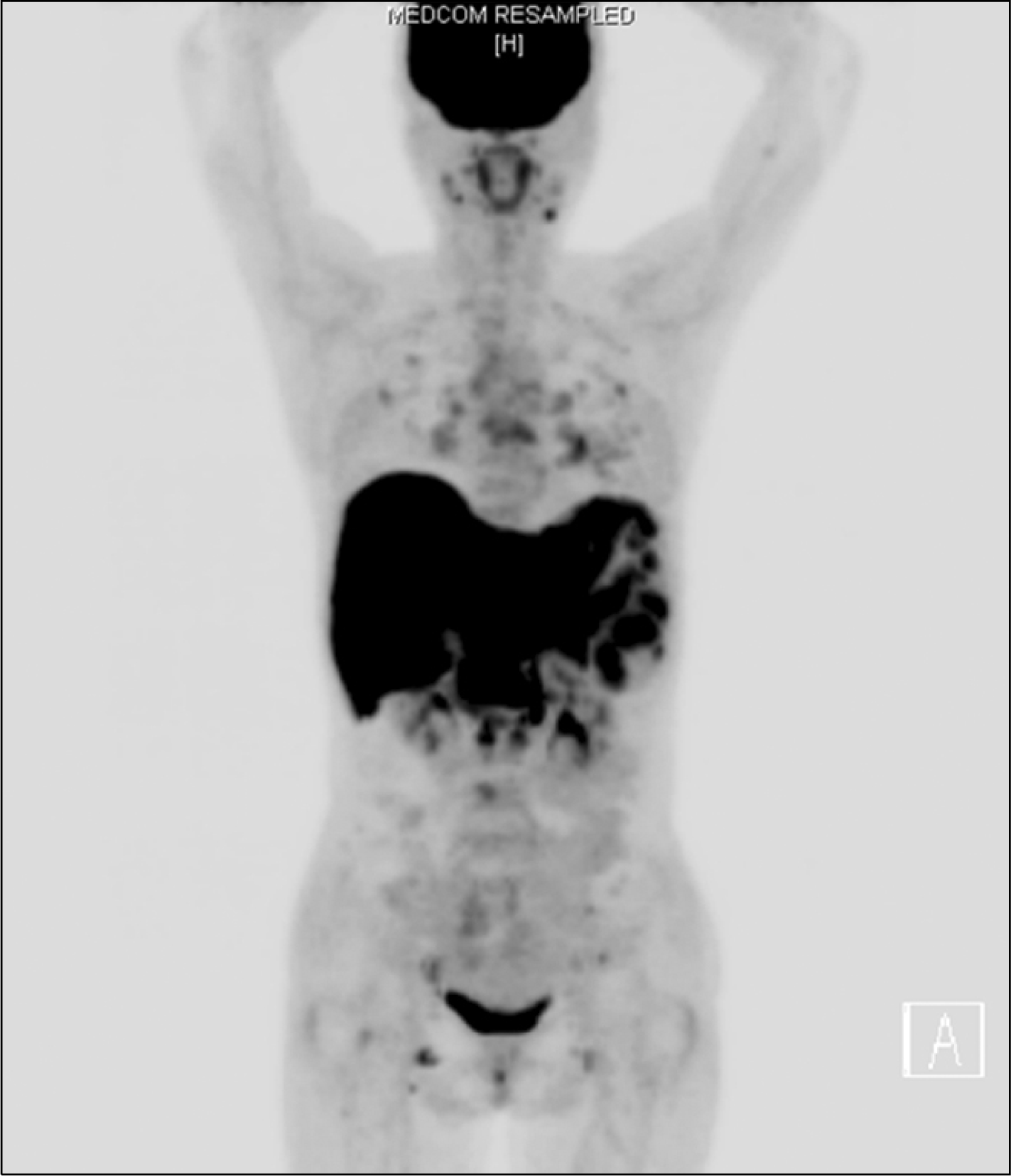
Figure 4.
Chest computed tomography (CT) scans (A, B) and abdominal CT scans (C, D). CT scans at the time of diagnosis (A, C) showing multiple perilymphatic nodules in both lungs (arrows) and heterogeneous enhancement in liver and spleen with hepatosplenomegaly. CT scans (B, D) taken after steroid treatment, revealing near total resolution of perilymphatic nodules and decreased size of liver and spleen.

Table 1.
Laboratory data
Ag: antigen, ALP: alkaline phosphatase, ALT: alanine transaminase, AST: aspartate transaminase, ESR: erythrocyte sedimentation rate, GT: glutamyl transpeptidase, HBe: hepatitis B e, HBs: hepatitis B surface, HBV: hepatitis B virus, HCV: hepatitis C virus, LDH: lactate dehydrogenase, RQ PCR: real-time quantitative polymerase chain reaction.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download