Abstract
Objectives
To investigate the clinical findings upon initial diagnosis and extraglandular manifestations in Korean patients with primary Sjögren's syndrome (pSS).
Methods
We collected clinical and laboratory data from 238 pSS patients enrolled at Seoul National University Hospital, Seoul National University Bundang Hospital and Seoul Medical Center from March 2011 to December 2014. All patients met the American-European Consensus Group criteria for pSS.
Results
Upon initial diagnosis, sicca symptoms (xerophthalmia or xerostomia) as the chief complaint were only observed in 129 (54.2%) pSS patients, while extraglandular manifestation was more common as the chief complaint in male patients or those with younger age (<40 years) than female or older patients (both p<0.05). Extraglandular manifestations were found in 178 (74.8%) patients, with musculoskeletal manifestations being most common (53.8%). Peripheral neuropathy in pSS patients was associated with Raynaud phenomenon and elevated serum total immunoglobulin G (IgG) levels (both p<0.05). Serum β2-microglobulin (β2-M) levels were significantly correlated with European League against Rheumatism (EULAR) Sjögren's syndrome disease activity index, erythrocyte sedimentation rate and serum total IgG (all p<0.001), and were higher in patients with extraglandular manifestations than those without (p<0.05). Serum C3 levels were decreased in patients with extraglandular manifestation, compared to those without (p<0.05). Malignant lymphoma was found in Korean pSS patients (1.7%) and associated with elevated serum β2-M levels (p<0.0001).
Go to : 
REFERENCES
1. Moutsopoulos HM. Sjögren's syndrome: autoimmune epithelitis. Clin Immunol Immunopathol. 1994; 72:162–5.
3. Baimpa E, Dahabreh IJ, Voulgarelis M, Moutsopoulos HM. Hematologic manifestations and predictors of lymphoma development in primary Sjögren syndrome: clinical and pathophysiologic aspects. Medicine (Baltimore). 2009; 88:284–93.
4. Voulgarelis M, Tzioufas AG, Moutsopoulos HM. Mortality in Sjögren's syndrome. Clin Exp Rheumatol. 2008; 26(5 Suppl 51):S66–71.
5. Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. European Study Group on Classification Criteria for Sjögren's Syndrome. Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002; 61:554–8.
6. Seror R, Ravaud P, Bowman SJ, Baron G, Tzioufas A, Theander E, et al. EULAR Sjögren's Task Force. EULAR Sjögren's syndrome disease activity index: development of a consensus systemic disease activity index for primary Sjögren's syndrome. Ann Rheum Dis. 2010; 69:1103–9.

7. Seo SH, Kim HS, Kwok SK, Ju JH, Kim SH, Yoon CH, et al. Extraglandular manifestations and autoantibodies of Korean patients with primary Sjögren's syndrome. J Korean Rheum Assoc. 2007; 14:43–50.

8. Baldini C, Talarico R, Tzioufas AG, Bombardieri S. Classification criteria for Sjögren's syndrome: a critical review. J Autoimmun. 2012; 39:9–14.
9. Goules AV, Tzioufas AG, Moutsopoulos HM. Classification criteria of Sjögren's syndrome. J Autoimmun. 2014; 48-49:42–5.

10. Venables PJ. Management of patients presenting with Sjögren's syndrome. Best Pract Res Clin Rheumatol. 2006; 20:791–807.
11. Rasmussen A, Ice JA, Li H, Grundahl K, Kelly JA, Radfar L, et al. Comparison of the American-European Consensus Group Sjögren's syndrome classification criteria to newly proposed American College of Rheumatology criteria in a large, carefully characterised sicca cohort. Ann Rheum Dis. 2014; 73:31–8.

12. García-Carrasco M, Ramos-Casals M, Rosas J, Pallarés L, Calvo-Alen J, Cervera R, et al. Primary Sjögren syndrome: clinical and immunologic disease patterns in a cohort of 400 patients. Medicine (Baltimore). 2002; 81:270–80.
13. Chai J, Logigian EL. Neurological manifestations of primary Sjögren's syndrome. Curr Opin Neurol. 2010; 23:509–13.
14. Nakamura H, Kawakami A, Hayashi T, Iwamoto N, Okada A, Tamai M, et al. Anti-centromere antibody-seropositive Sjögren's syndrome differs from conventional subgroup in clinical and pathological study. BMC Musculoskelet Disord. 2010; 11:140.

15. Bournia VK, Diamanti KD, Vlachoyiannopoulos PG, Moutsopoulos HM. Anticentromere antibody positive Sjögren's syndrome: a retrospective descriptive analysis. Arthritis Res Ther. 2010; 12:R47.

16. Parke AL. Pulmonary manifestations of primary Sjögren's syndrome. Rheum Dis Clin North Am. 2008; 34:907–20.

17. Ito I, Nagai S, Kitaichi M, Nicholson AG, Johkoh T, Noma S, et al. Pulmonary manifestations of primary Sjögren's syndrome: a clinical, radiologic, and pathologic study. Am J Respir Crit Care Med. 2005; 171:632–8.
18. Ebert EC. Gastrointestinal and hepatic manifestations of Sjögren syndrome. J Clin Gastroenterol. 2012; 46:25–30.
19. Gottenberg JE, Busson M, Cohen-Solal J, Lavie F, Abbed K, Kimberly RP, et al. Correlation of serum B lymphocyte stimulator and beta2 microglobulin with autoantibody secretion and systemic involvement in primary Sjögren's syndrome. Ann Rheum Dis. 2005; 64:1050–5.
20. Gottenberg JE, Seror R, Miceli-Richard C, Benessiano J, Devauchelle-Pensec V, Dieude P, et al. Serum levels of be-ta2-microglobulin and free light chains of immunoglobulins are associated with systemic disease activity in primary Sjögren's syndrome. Data at enrollment in the prospective ASSESS cohort. PLoS One. 2013; 8:e59868.

21. Pertovaara M, Korpela M. Serum β 2 microglobulin correlates with the new ESSDAI in patients with Sjögren's syndrome. Ann Rheum Dis. 2011; 70:2236–7.
22. Zulman J, Jaffe R, Talal N. Evidence that the malignant lymphoma of Sjögren's syndrome is a monoclonal B-cell neoplasm. N Engl J Med. 1978; 299:1215–20.

23. Theander E, Manthorpe R, Jacobsson LT. Mortality and causes of death in primary Sjögren's syndrome: a prospective cohort study. Arthritis Rheum. 2004; 50:1262–9.

24. Pertovaara M, Pukkala E, Laippala P, Miettinen A, Pasternack A. A longitudinal cohort study of Finnish patients with primary Sjögren's syndrome: clinical, immunological, and epidemiological aspects. Ann Rheum Dis. 2001; 60:467–72.
Go to : 
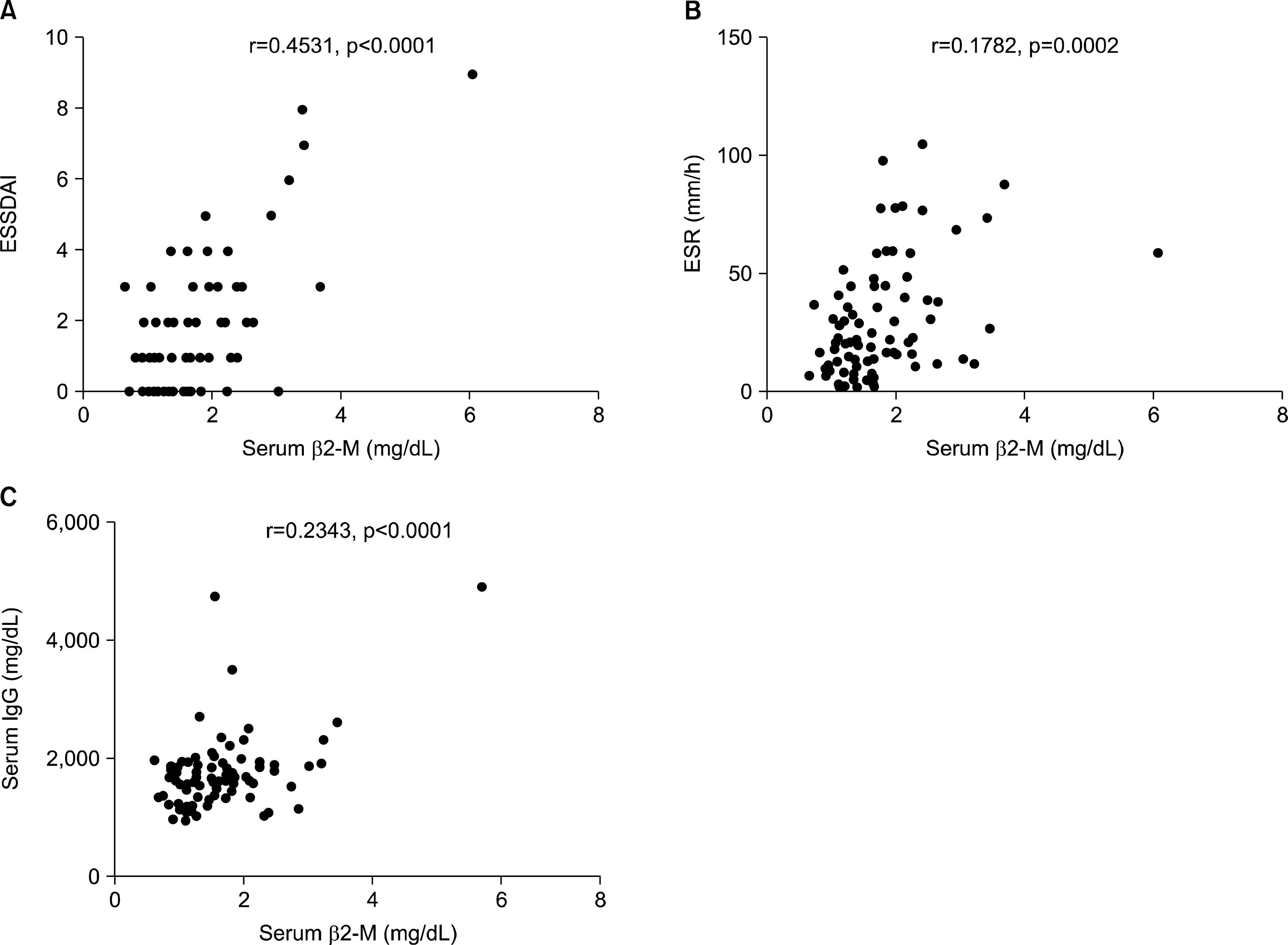 | Figure 1.The correlation of serum β2-microglobulin (β2-M) concentration with European League against Rheumatism (EULAR) Sjögren' s Syndrome Disease Activity Index (ESSDAI) (A), erythrocyte sediment rate (ESR) (B) and serum immunoglobulin G (IgG) (C) in 238 patients with primary Sjögren' s syndrome. |
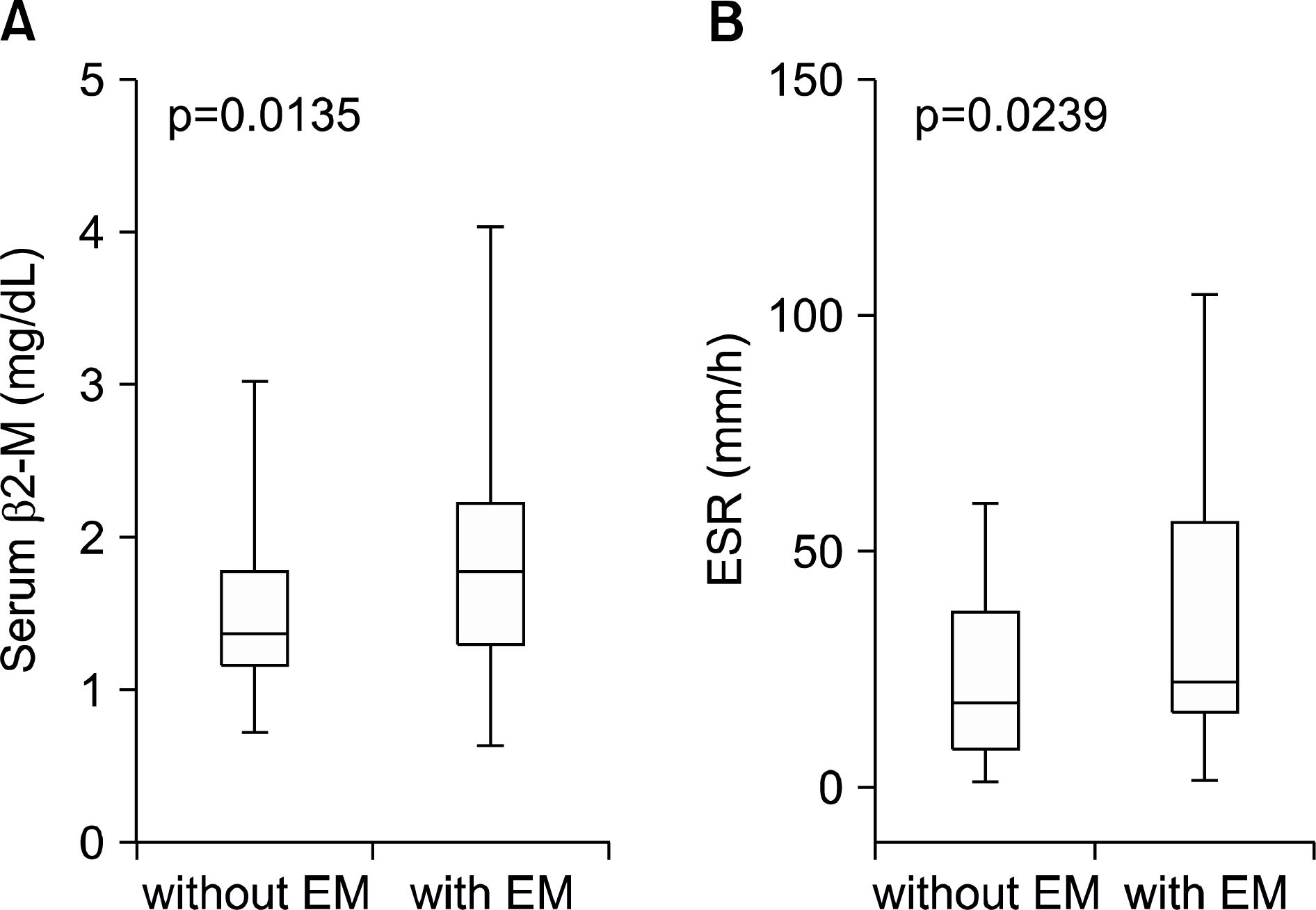 | Figure 2.The comparison of serum β2-microglobulin (β2-M) levels (A) and erythrocyte sediment rate (ESR) (B) between primary Sjögren' s syndrome patients with and without extraglandular manifestation (EM). |
Table 1.
Chief complaints at diagnosis in 238 patients with primary Sjögren's syndrome
Table 2.
The comparison of clinical and laboratory findings according to chief complaints at diagnosis in 238 patients with primary Sjögren's syndrome
| Variable | Sicca symptom (n=129) | Extraglandular manifestation (n=109) | p-value‡ |
|---|---|---|---|
| Xerophthalmia | 123 (95.3) | 98 (89.9) | 0.1313 |
| Positive Schirmer I test | 83 (64.3) | 54 (49.5) | 0.0254 |
| Xerostomia | 115 (89.1) | 92 (84.4) | 0.3351 |
| Salivary gland involvement* | 112 (86.8) | 79 (72.5) | 0.0085 |
| Musculoskeletal involvement | 71 (55.0) | 57 (52.3) | 0.6970 |
| Raynaud phenomenon | 23 (17.8) | 31 (28.4) | 0.0624 |
| Abnormal chest radiograph | 3 (2.3) | 19 (17.4) | <0.0001 |
| Abnormal liver function test | 2 (1.6) | 9 (8.3) | 0.0257 |
| Positive anti-nuclear antibody | 114 (88.4) | 91 (83.5) | 0.3470 |
| Positive rheumatoid factor | 85 (65.9) | 79 (72.5) | 0.3225 |
| Positive anti-Ro/SSA | 110 (85.3) | 88 (80.7) | 0.3871 |
| Positive anti-La/SSB | 82 (63.6) | 60 (55.0) | 0.1880 |
| Histopathology of minor salivary glands† | 25/42 (59.5) | 34/51 (66.7) | 0.5213 |
Table 3.
Cumulative clinical and laboratory findingsin 238 patients with primary Sjögren's syndrome defined by revised European criteria proposed by the American-European Consensus Group
| Variable | Present study (n=238) | Seo et al. [7] (n=125) |
|---|---|---|
| Xerophthalmia | 221 (92.9) | 122 (97.6) |
| Positive Schirmer I test | 137 (57.6) | 114 (91.2) |
| Xerostomia | 213 (89.5) | 123 (98.4) |
| Salivary gland involvement* | 191 (80.3) | 109/116 (93.6) |
| Musculoskeletal involvement | 128 (53.8) | NA |
| Arthralgia/arthritis | 114 (47.9) | 82 (65.6) |
| Myalgia | 68 (28.6) | 46 (36.8) |
| Raynaud phenomenon | 54 (22.7) | 30 (24.0) |
| Dry skin | 48 (20.2) | 24 (19.2) |
| Skin rash | 23 (9.7) | 7 (5.6) |
| Vasculitis | 14 (5.9) | 5 (4.0) |
| Central nervous system disease | 11 (4.6) | 8 (6.4) |
| Peripheral neuropathy | 23 (9.7) | 29 (23.2) |
| Lung nodule | 7 (2.9) | 6 (4.8) |
| Interstitial lung disease | 15 (6.3) | 6 (4.8) |
| Hepatobiliary involvement | 12 (5.0) | 7 (5.6) |
| Renal tubular acidosis | 9 (3.8) | 2 (1.6) |
| Hypothyroidism | 29 (12.2) | 16 (12.8) |
| Hyperthyroidism | 10 (4.2) | 4 (3.2) |
| Malignant lymphoma | 4 (1.7) | 2 (1.6) |
| Leukopenia | 78 (32.8) | NA |
| Thrombocytopenia | 6 (2.5) | NA |
| Positive anti-nuclear antibody | 205 (86.1) | 101 (80.8) |
| Positive rheumatoid factor | 164 (68.9) | 99 (79.2) |
| Positive anti-Ro/SSA | 198 (83.2) | 90 (72.0) |
| Positive anti-La/SSB | 142 (59.7) | 38 (30.4) |
| Positive anti-Ro/SSA or anti-La/SSB | 209 (87.8) | NA |
| Histopathology of minor salivary glands† | 59/93 (63.4) | 27/34 (75) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download