Abstract
Objective
Although several ginsenosides have been reported to have anti-arthritic activity, few in vivo studies of the anti-ar-thritic effects of compound K (CK), a major metabolite of ginsenosides, have been conducted. Therefore, we investigated the preventative and therapeutic effects of CK on collagen-induced arthritis (CIA).
Methods
CK was administered to CIA mice pre-ventively and therapeutically and post-treatment bone microarchitectural characteristics, histopathological changes, and serum levels of anti-collagen antibodies, tumor necrosis factor-α, and interleukin (IL)-17 were investigated. We also examined cytokine production by type II collagen (CII)-stimulated splenocytes and mRNA expression of matrix metalloproteinases (MMPs), tissue inhibitors of metalloproteinase (TIMP)-1, receptor activator of nuclear factor-κ B ligand (RANKL), and osteoprotegerin (OPG) in the joint tissues.
Results
CK reduced the severity of CIA preventively and therapeutically (all p<0.05). Additionally, CK dose-dependently decreased histopathological signs of arthritis and improved microarchitectural characteristics (all p <0.05) at 10 to 20 mg/kg/d in CIA mice. CK treatment significantly decreased the serum levels of anti-CII immunoglobulin G (p<0.01) and the secretion of interferon-γ and IL-2 from stimulated splenocytes (all p<0.05). Furthermore, MMP-3/TIMP-1 and RANKL/OPG ratios were suppressed in CK treated mice (all p<0.01).
Go to : 
REFERENCES
1. Furneri G, Mantovani LG, Belisari A, Mosca M, Cristiani M, Bellelli S, et al. Systematic literature review on economic implications and pharmacoeconomic issues of rheumatoid arthritis. Clin Exp Rheumatol. 2012; 30(4 Suppl 73):S72–84.
2. Smolen JS, Aletaha D, Bijlsma JW, Breedveld FC, Boumpas D, Burmester G, et al. T2T Expert Committee. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010; 69:631–7.

3. Jung YO, Kim HA. Recent paradigm shifts in the diagnosis and treatment of rheumatoid arthritis. Korean J Intern Med. 2012; 27:378–87.

4. Moreland L. Unmet needs in rheumatoid arthritis. Arthritis Res Ther. 2005; 7(Suppl 3):S2–8.
5. Kim HA, Kim S, Chang SH, Hwang HJ, Choi YN. Anti-ar-thritic effect of ginsenoside Rb1 on collagen induced arthritis in mice. Int Immunopharmacol. 2007; 7:1286–91.

6. Chang SH, Choi Y, Park JA, Jung DS, Shin J, Yang JH, et al. Anti-inflammatory effects of BT-201, an n-butanol extract of Panax notoginseng, observed in vitro and in a collagen-induced arthritis model. Clin Nutr. 2007; 26:785–91.

7. Shin JS, Park N, Ra J, Kim Y, Shin M, Hong M, et al. Panax ginseng C.A. Meyer modulates the levels of MMP3 in S12 murine articular cartilage cell line. J Ethnopharmacol. 2009; 124:397–403.

8. Kim KR, Chung TY, Shin H, Son SH, Park KK, Choi JH, et al. Red ginseng saponin extract attenuates murine collagen-induced arthritis by reducing proinflammatory responses and matrix metalloproteinase-3 expression. Biol Pharm Bull. 2010; 33:604–10.

9. Du J, Cheng B, Zhu X, Ling C. Ginsenoside Rg1, a novel glucocorticoid receptor agonist of plant origin, maintains glucocorticoid efficacy with reduced side effects. J Immunol. 2011; 187:942–50.

10. Na JY, Kim S, Song K, Lim KH, Shin GW, Kim JH, et al. Anti-apoptotic activity of ginsenoside Rb1 in hydrogen peroxide-treated chondrocytes: stabilization of mitochondria and the inhibition of caspase-3. J Ginseng Res. 2012; 36:242–7.
11. Kim S, Na JY, Song KB, Choi DS, Kim JH, Kwon YB, et al. Protective effect of ginsenoside Rb1 on hydrogen pero-xide-induced oxidative stress in rat articular chondrocytes. J Ginseng Res. 2012; 36:161–8.
12. Cheng W, Wu D, Zuo Q, Wang Z, Fan W. Ginsenoside Rb1 prevents interleukin-1 beta induced inflammation and apoptosis in human articular chondrocytes. Int Orthop. 2013; 37:2065–70.

13. So MW, Lee EJ, Lee HS, Koo BS, Kim YG, Lee CK, et al. Protective effects of ginsenoside Rg3 on human osteoarthritic chondrocytes. Mod Rheumatol. 2013; 23:104–11.

14. Lee JH, Lim H, Shehzad O, Kim YS, Kim HP. Ginsenosides from Korean red ginseng inhibit matrix metalloproteinase-13 expression in articular chondrocytes and prevent cartilage degradation. Eur J Pharmacol. 2014; 724:145–51.

15. Yu K, Chen F, Li C. Absorption, disposition, and pharmacokinetics of saponins from Chinese medicinal herbs: what do we know and what do we need to know more? Curr Drug Metab. 2012; 13:577–98.

16. Choi YS, Kang EH, Lee EY, Gong HS, Kang HS, Shin K, et al. Joint-protective effects of compound K, a major ginsenoside metabolite, in rheumatoid arthritis: in vitro evidence. Rheumatol Int. 2013; 33:1981–90.

17. Wu H, Chen J, Wang Q, Jia X, Song S, Yuan P, et al. Ginsenoside metabolite compound K attenuates inflammatory responses of adjuvant-induced arthritis rats. Immunopharmacol Immunotoxicol. 2014; 36:124–9.

18. Liu KK, Wang QT, Yang SM, Chen JY, Wu HX, Wei W. Ginsenoside compound K suppresses the abnormal activation of T lymphocytes in mice with collagen-induced arthritis. Acta Pharmacol Sin. 2014; 35:599–612.

19. Chen J, Wu H, Wang Q, Chang Y, Liu K, Song S, et al. Ginsenoside metabolite compound K alleviates adjuvantinduced arthritis by suppressing T cell activation. Inflammation. 2014; 37:1608–15.

20. Kim HO, Lee SI. Experimental animal models for rheumatoid arthritis: methods and applications. J Rheum Dis. 2012; 19:189–95.

21. Zhou W, Feng MQ, Li JY, Zhou P. Studies on the preparation, crystal structure and bioactivity of ginsenoside compound K. J Asian Nat Prod Res. 2006; 8:519–27.

23. Wooley PH. Collagen-induced arthritis in the mouse. Methods Enzymol. 1988; 162:361–73.
24. Xiao J, Shimada M, Liu W, Hu D, Matsumori A. Anti-inflammatory effects of eplerenone on viral myocarditis. Eur J Heart Fail. 2009; 11:349–53.

25. Wells JE, Rice TK, Nuttall RK, Edwards DR, Zekki H, Rivest S, et al. An adverse role for matrix metalloproteinase 12 after spinal cord injury in mice. J Neurosci. 2003; 23:10107–15.

26. Palmqvist P, Lundberg P, Persson E, Johansson A, Lundgren I, Lie A, et al. Inhibition of hormone and cytokine-stimulated osteoclastogenesis and bone resorption by interleukin-4 and interleukin-13 is associated with increased osteoprotegerin and decreased RANKL and RANK in a STAT6-dependent pathway. J Biol Chem. 2006; 281:2414–29.

27. Lee JH, Chun KJ, Kim HS, Kim SH, Lee KY, Kim DJ, et al. Changes in microarchitectural characteristics at the tibial epiphysis induced by collagen-induced rheumatoid arthritis over time. Clin Interv Aging. 2012; 7:373–82.
28. Nandakumar KS, Bäcklund J, Vestberg M, Holmdahl R. Collagen type II (CII)-specific antibodies induce arthritis in the absence of T or B cells but the arthritis progression is enhanced by CII-reactive T cells. Arthritis Res Ther. 2004; 6:R544–50.
29. Matsuda H, Samukawa K, Kubo M. Anti-inflammatory activity of ginsenoside Ro. Planta Med. 1990; 56:19–23.
31. Kim KA, Jung IH, Park SH, Ahn YT, Huh CS, Kim DH. Comparative analysis of the gut microbiota in people with different levels of ginsenoside Rb1 degradation to compound K. PLoS One. 2013; 8:e62409.

32. Kim HK. Pharmacokinetics of ginsenoside Rb1 and its metabolite compound K after oral administration of Korean Red Ginseng extract. J Ginseng Res. 2013; 37:451–6.

33. Gao YL, Liu ZF, Li CM, Shen JY, Yin HX, Li GS. Subchronic toxicity studies with ginsenoside compound K delivered to dogs via intravenous administration. Food Chem Toxicol. 2011; 49:1857–62.

34. Rowley MJ, Nandakumar KS, Holmdahl R. The role of collagen antibodies in mediating arthritis. Mod Rheumatol. 2008; 18:429–41.

35. Germann T, Bongartz M, Dlugonska H, Hess H, Schmitt E, Kolbe L, et al. Interleukin-12 profoundly upregulates the synthesis of antigen-specific complement-fixing IgG2a, IgG2b and IgG3 antibody subclasses in vivo. Eur J Immunol. 1995; 25:823–9.
36. Terato K, Hasty KA, Reife RA, Cremer MA, Kang AH, Stuart JM. Induction of arthritis with monoclonal antibodies to collagen. J Immunol. 1992; 148:2103–8.
37. Billiau A, Matthys P. Collagen-induced arthritis and related animal models: how much of their pathogenesis is autoimmune, how much is autoinflammatory? Cytokine Growth Factor Rev. 2011; 22:339–44.

38. Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003; 171:6173–7.

39. Mori L, Iselin S, De Libero G, Lesslauer W. Attenuation of collagen-induced arthritis in 55-kDa TNF receptor type 1 (TNFR1)-IgG1-treated and TNFR1-deficient mice. J Immunol. 1996; 157:3178–82.
40. Lee ES, Choi JS, Kim MS, You HJ, Ji GE, Kang YH. Ginsenoside metabolite compound K differentially antag-onizing tumor necrosis factor-α-induced monocyte-endothelial trafficking. Chem Biol Interact. 2011; 194:13–22.

41. Choi K, Kim M, Ryu J, Choi C. Ginsenosides compound K and Rh(2) inhibit tumor necrosis factor-alpha-induced activation of the NF-kappaB and JNK pathways in human as-troglial cells. Neurosci Lett. 2007; 421:37–41.
Go to : 
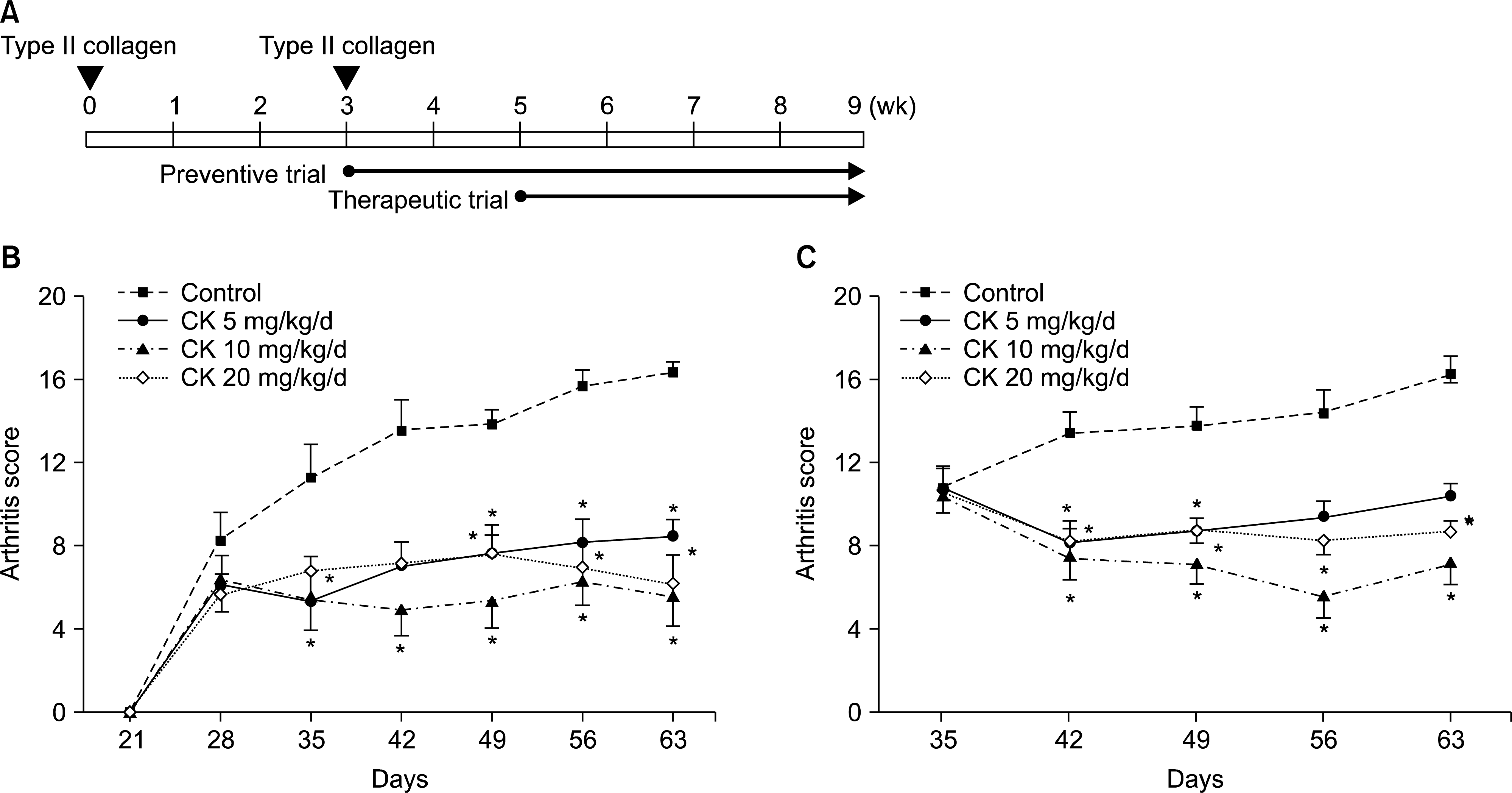 | Figure 1.The effect of compound K (CK) on collagen-induced arthritis (CIA) disease activity. CK (0, 5, 10, or 20 mg/kg/d) was administered 1 day (preventive trial) or 14 days (therapeutic trial) after the boosting immunization of type II collagen (A). Preventive (B, n=9) and therapeutic (C, n=9) administration of CK significantly decreased arthritis scores along the disease course. However, the suppressive effects of CK were not dose-dependent in the dose range tested (5 to 20 mg/kg/d). Error bars represent the standard error of the mean. *p<0.05 by Kruskal-Wallace test with Dunn's multiple comparison test. |
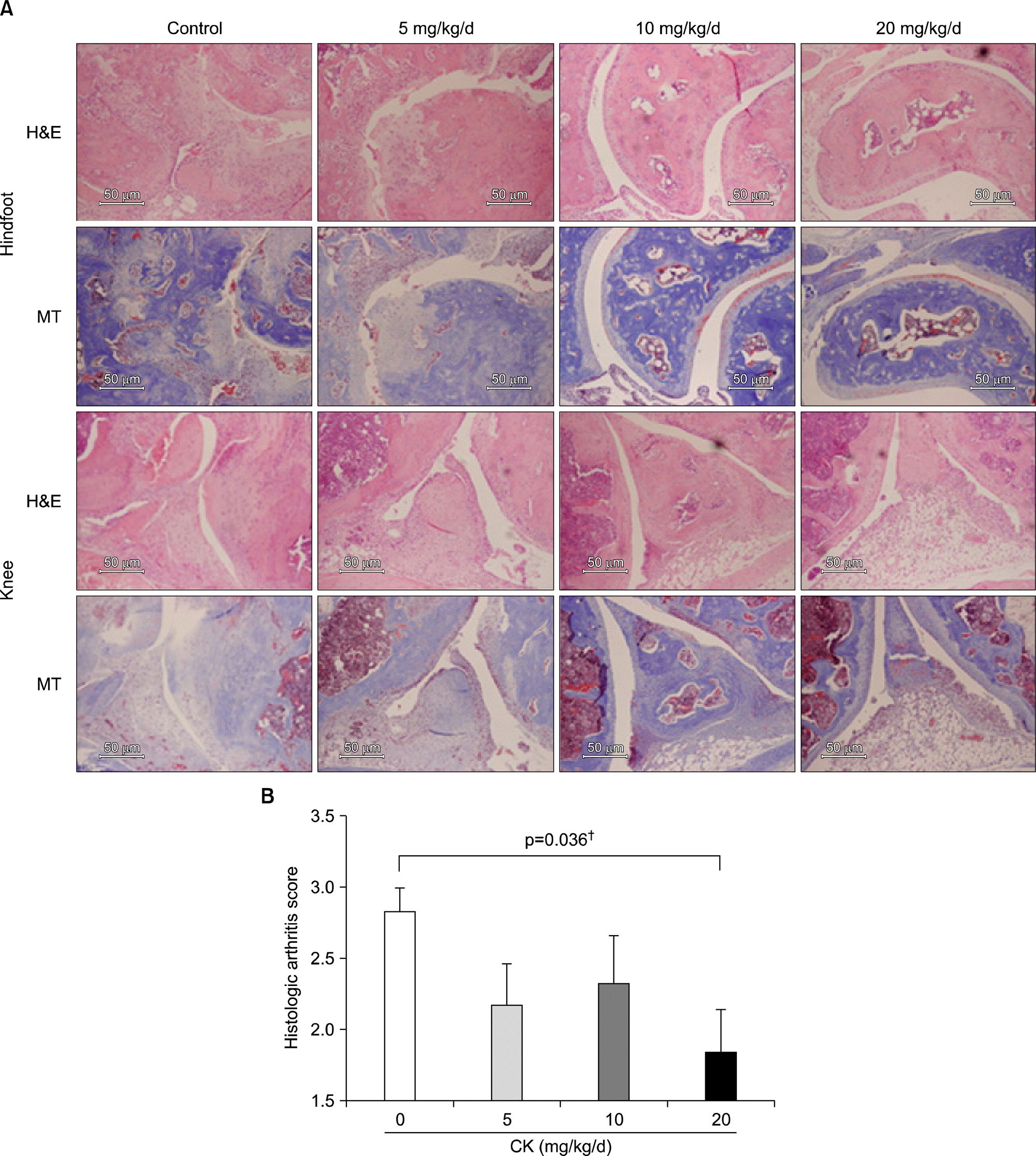 | Figure 2.Representative sections of joint tissues (hindfoot and knee joints) stained with hematoxylin-eosin (H&E) and Masson's Trichrome (MT) in therapeutic models (scale bar, 50 μ m; A). Control group showed typical findings of collagen-induced arthritis (CIA); severe inflammatory cells infiltration, synovial hypertrophy, joint space narrowing, and bone and cartilage damage. These arthritic findings were decreased in mice therapeutically administered with compound K (CK). The histologic arthritis scores in knee joints significantly decreased with increasing dose of CK (B, n=6). Error bars represent the standard error of the mean. † p-val-ue was calculated by Jonckheere's trend test. |
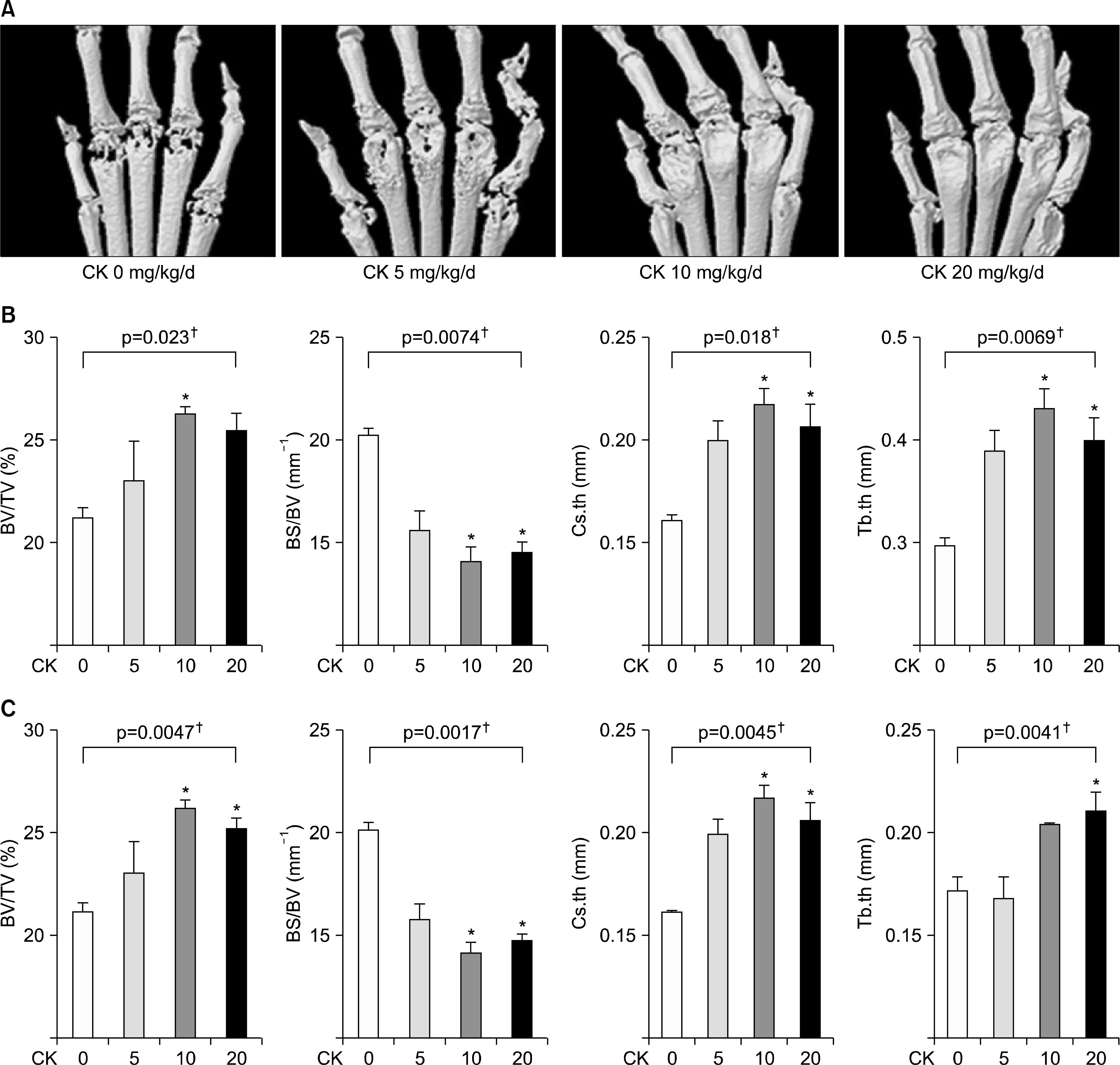 | Figure 3.Microarchitectural analysis using micro-computed tomography (micro-CT) in therapeutic models (n=4 in each group, respectively). Representative micro-CT images of the hind paws (A) showed that the erosion in the metatarsophalangeal joints was decreased in compound K (CK)-treated groups. (B, C) Microarchitectural parameters included bone volume/total tissue volume (BV/TV), bone surface/bone volume (BS/BV), cross-sectional thickness (Cs.th), and trabecular thickness (Tb.th). Therapeutic CK administration significantly increased BV/TV, Cs.th, and Tb.th values and significantly decreased BS/BV values. Error bars represent the standard error of the mean. *p<0.05 by Kruskal-Wallace test with Dunn's multiple comparison test; †p-values were calculated by Kruskal-Wallis test. |
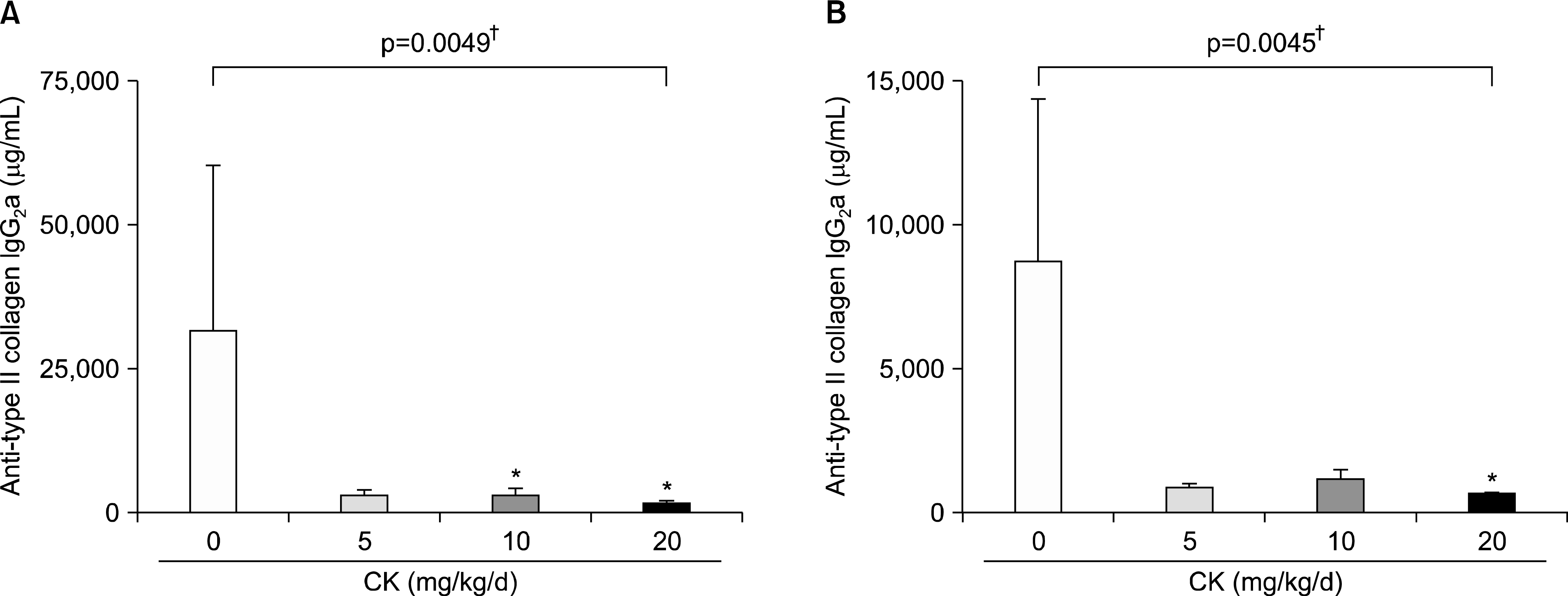 | Figure 4.Serum levels of anti-type II collagen (CII) antibodies in therapeutic models. Therapeutical administration of compound K (CK) produced a significant reduction of anti-CII antibody IgG2 a (A) and IgG2 b (B, n=6 in each group). Error bars represent the standard error of the mean. *p<0.05 by Kruskal-Wallace test with Dunn's multiple comparison test; † p-values were calculated by Kruskal-Wallis test. |
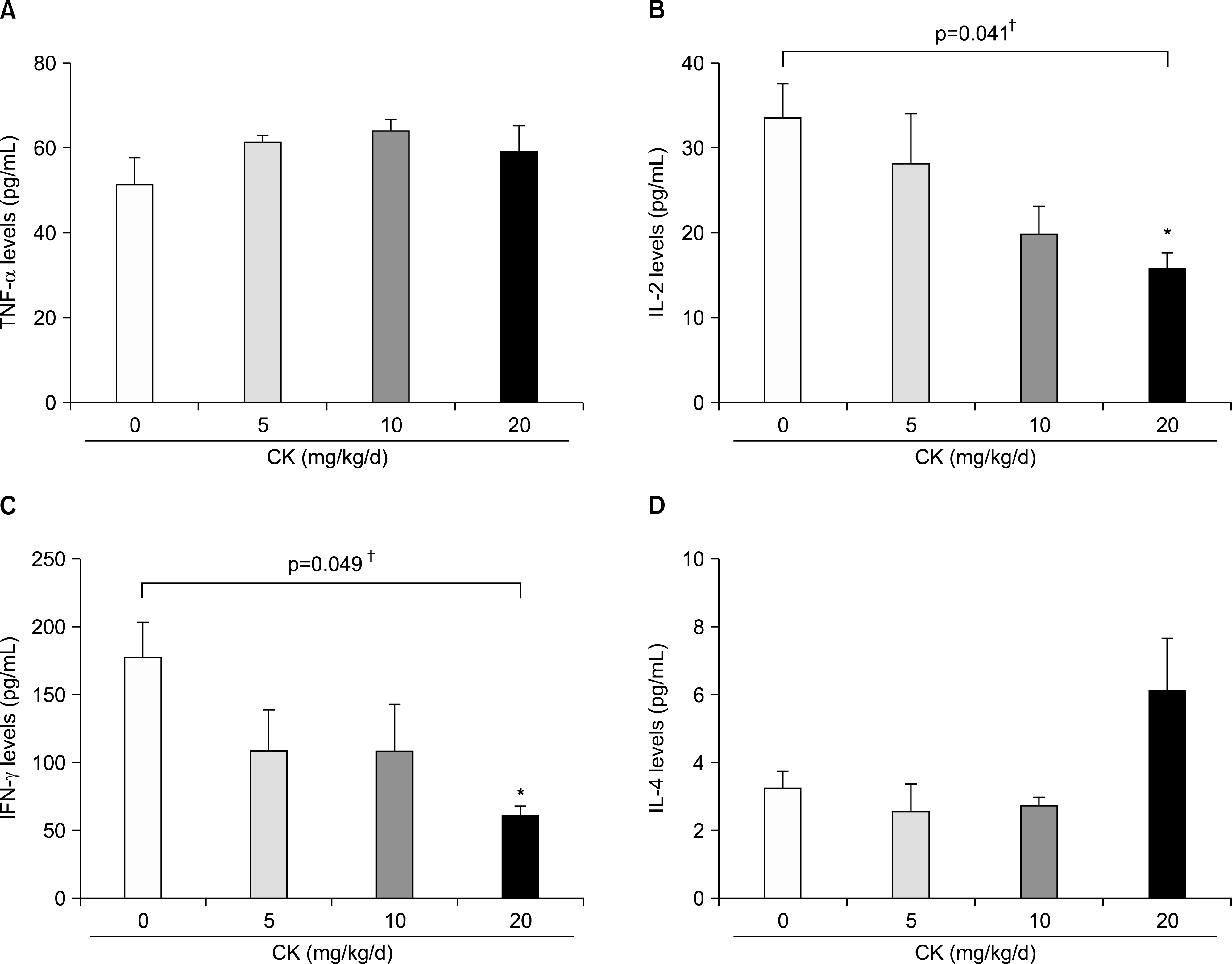 | Figure 5.Cytokine production from the type II collagen stimulated splenocytes in therapeutic models. Splenocytes were isolated at the time of sacrifice after 4 weeks of compound K (CK) treatment and were stimulated with bovine CII for 48 h (n=6 in each group). When tumor necrosis factor (TNF)-α (A), interleukin (IL)-2 (B), interferon (IFN)-γ (C), and IL-4 (D) levels were measured in the culture media, IL-2 and IFN-γ were dose-dependently decreased in CK-treated groups. However, no significant effects on TNF-α or IL-4 production were observed. Error bars represent the standard error of the mean. *p<0.05 by Kruskal-Wallace test with Dunn's multiple comparison test; †p-values were calculated by Kruskal-Wallis test. |
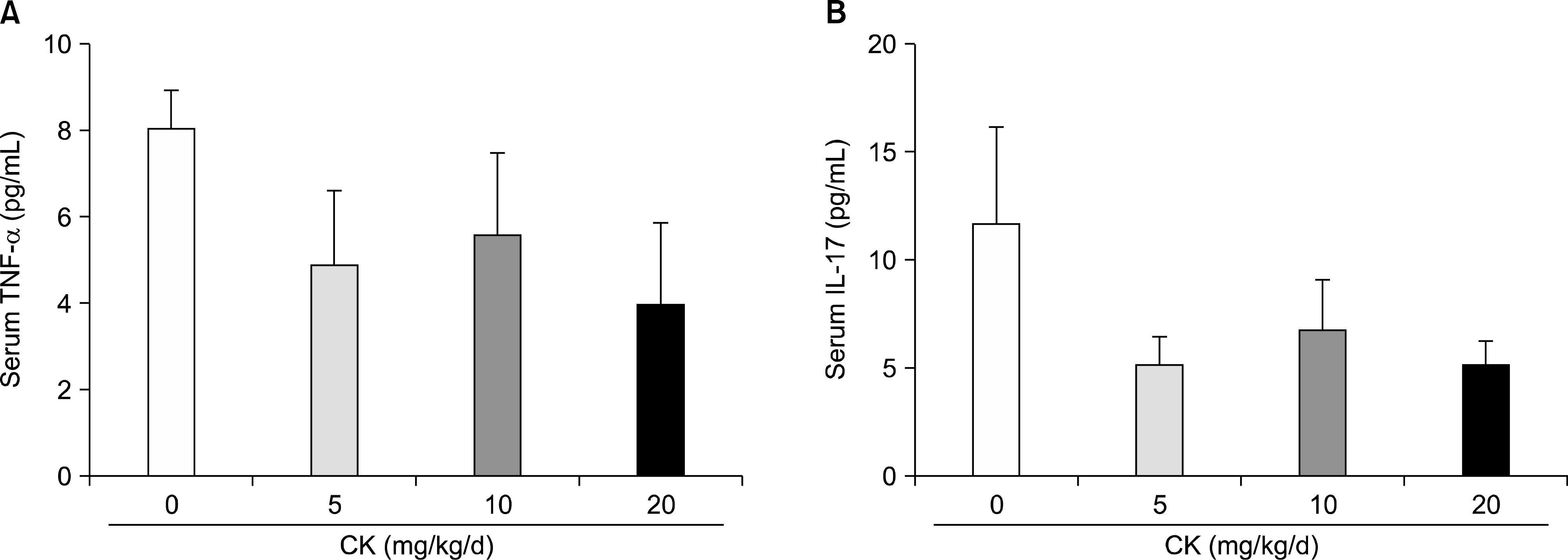 | Figure 6.Serum levels of tumor necrosis factor (TNF)-α and interleukin (IL)-17 in therapeutic models (n=6 in each group). Therapeutic compound K (CK) administration did not significantly affect TNF-α (A) and IL-17 (B) levels. Error bars represent the standard error of the mean. |
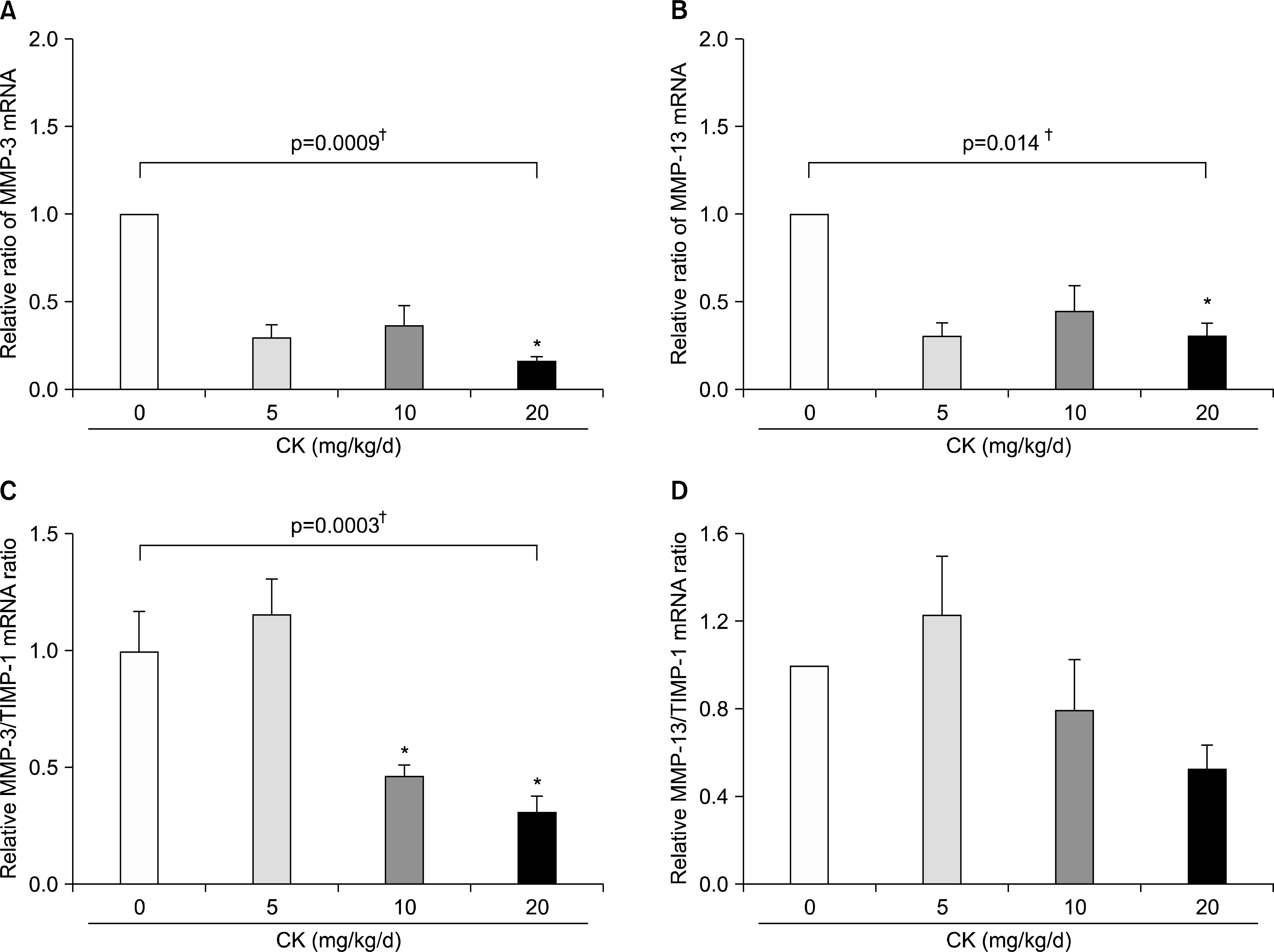 | Figure 7.Effects of compound K (CK) on matrix metalloproteinase (MMP)-3 and MMP-13 mRNA expression in the hind feet of therapeutic models. When MMP-3 and MMP-13 mRNA levels in the hind foot tissue were analyzed using quantitative real-time polymerase chain reaction (n=6 in each group), therapeutic administration of CK significantly decreased expression of MMP-3 (A) and MMP-13 (B). The suppressive effect of CK on MMP-3 expression remained significant after adjustment for tissue inhibitors of metalloproteinase (TIMP)-1 mRNA (C, n=4 in each group). MMP-13/TIMP-1 ratios (D, n=4 in each group) tended to decrease in a dose dependent manner. Error bars represent the standard error of the mean. *p<0.05 by Kruskal-Wallace test with Dunn's multiple comparison test; † p-values were calculated by Kruskal-Wallis test. |
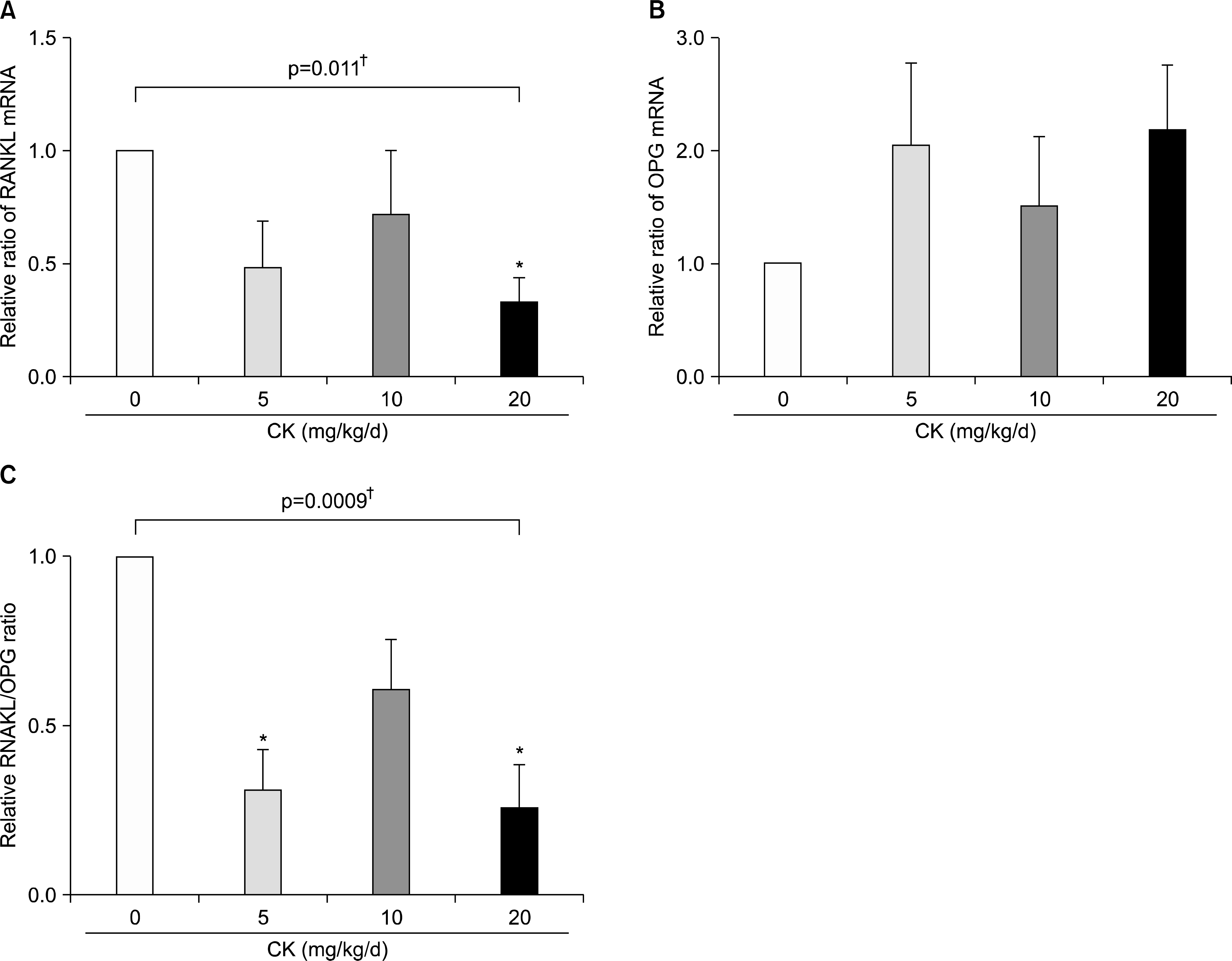 | Figure 8.Effects of compound K (CK) on receptor activator of nuclear factor-κ B ligand (RANKL) and osteoprotegerin (OPG) mRNA expression in the hind feet of therapeutic models. When RANKL and OPG mRNA levels in the hind foot tissue were analyzed using quantitative real-time polymerase chain reaction (n=6 in each group), therapeutic CK administration significantly decreased RANKL mRNA expression (A) and tended to increase OPG mRNA (B). Consequently, the ratios of RANKL/OPG were significantly reduced in CK administered mice (C). Error bars represent the standard error of the mean. *p<0.05 by Kruskal-Wallace test with Dunn's multiple comparison test; † p-values were calculated by Kruskal-Wallis test. |
Table 1.
Primers and probes used for quantitative real-time polymerase chain reaction




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download