Abstract
Tjalma or pseudo-pseudo Meigs' syndrome is a clinical condition that is characterized with ascites, pleural effusion, and increased serum CA-125 levels in patients with systemic lupus erythematosus (SLE) without the presence of ovarian tumor. On the other hand, Meigs' and pseudo-Meigs' syndromes represent the same manifestations with ovarian tumor. In this case report, we present a 43-year-old SLE patient suffering from Tjalma syndrome with the coexistence of incidental ovarian teratoma, who was successfully treated with intravenous immunoglobulin-G adjunctive therapy after inadequate response to surgical excision of the ovarian tumor, steroid, and cyclophosphamide pulse therapy.
Go to : 
REFERENCES
1. Meigs JV, Cass JW. Fibroma of the ovary with ascites and hydrothorax: with a report of seven cases. Am J Obstet Gynecol. 1937; 33:249–66.
2. Nagakura S, Shirai Y, Hatakeyama K. Pseudo-Meigs' syndrome caused by secondary ovarian tumors from gastrointestinal cancer. A case report and review of the literature. Dig Surg. 2000; 17:418–9.
4. Schmitt R, Weichert W, Schneider W, Luft FC, Kettritz R. Pseudo-pseudo Meigs' syndrome. Lancet. 2005; 366:1672.

5. Tjalma WA. Ascites, pleural effusion, and CA 125 elevation in an SLE patient, either a Tjalma syndrome or, due to the migrated Filshie clips, a pseudo-Meigs syndrome. Gynecol Oncol. 2005; 97:288–91.

6. Ural UM, Kiliç A, Gü ngör T, Ozdal B, Mollamahmutoğlu L. Tjalma's or pseudo-pseudo-Meigs' syndrome: a case report. Clin Exp Dermatol. 2008; 33:363–4.

7. Bes C, DağlıÜ , Memedoğlu P, Soy M. A rare form of SLE: pseudo-pseudo meigs syndrome and hydrocephalus. Rheumatol Int. 2013; 33:2175–6.

8. Dalvi SR, Yildirim R, Santoriello D, Belmont HM. Pseudo-pseudo Meigs' syndrome in a patient with systemic lupus erythematosus. Lupus. 2012; 21:1463–6.

9. Prasad S, Abujam B, Lawrence A, Aggarwal A. Massive ascites as a presenting feature of lupus. Int J Rheum Dis. 2012; 15:e15–6.

10. Abramov Y, Anteby SO, Fasouliotis SJ, Barak V. The role of inflammatory cytokines in Meigs' syndrome. Obstet Gynecol. 2002; 99:917–9.

11. Yang Z, Liang Y, Li C, Zhong R. Serum CA125 elevation is independently associated with serositis in SLE patients. Clin Exp Rheumatol. 2012; 30:93–8.
Go to : 
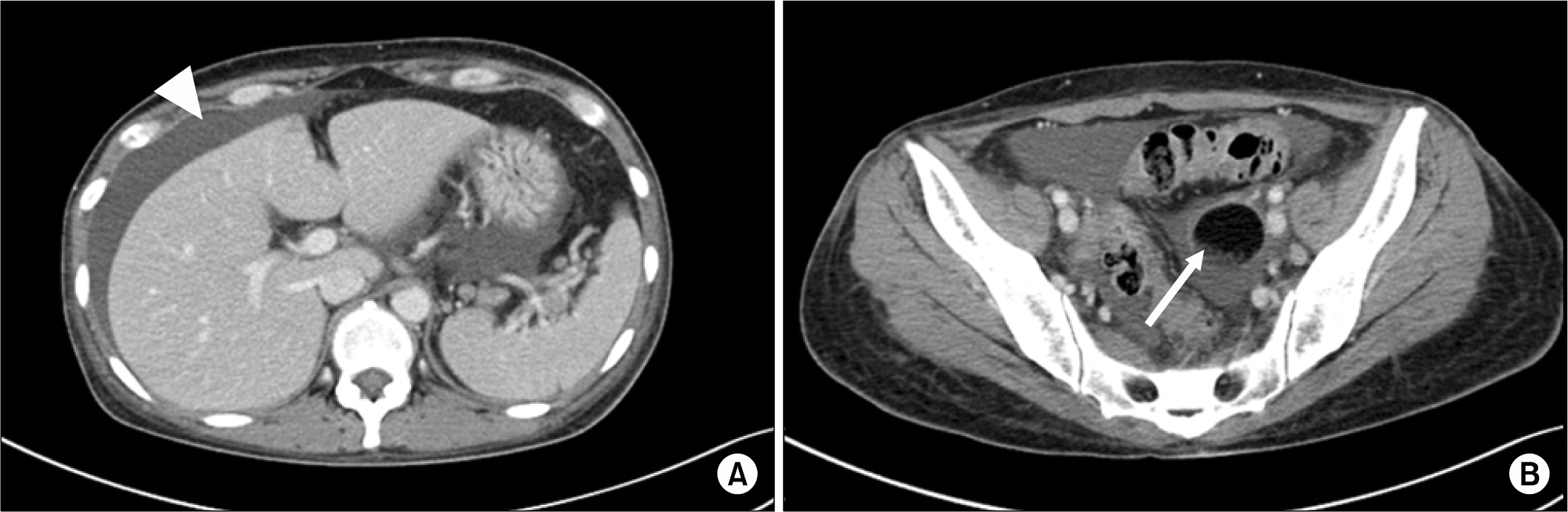 | Figure 2.Abdominal computed tomography showing ascites (arrowhead in A) and 4.3 cm sized ovarian tumor (arrow in B). |
Table 1.
Changes in serum CA-125 and albumin concentrations according to therapy




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download