Abstract
Objective
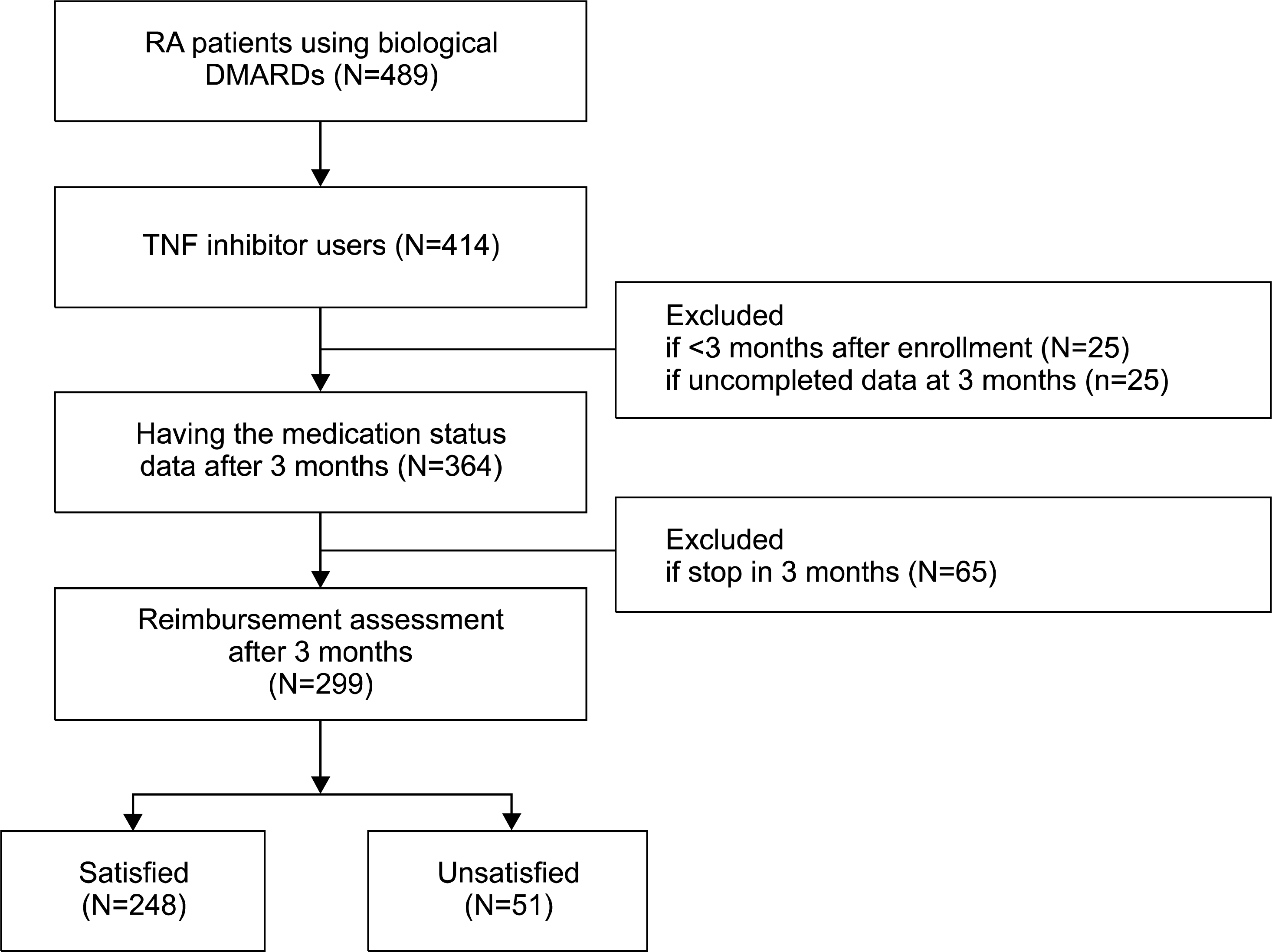
The purpose of this study is to examine the difference between the numbers of patients in rheumatoid arthritis (RA) who are eligible to TNF inhibitors by the past Korean National Health Insurance reimbursement guideline and by the disease activity score with 28-joint assessment (DAS28) based criteria.
Methods
Data were obtained from a multicenter registry for biologics users in Korean RA patients, BIOlogics Phar-macoepidemiologic StudY (BIOPSY). DAS28 was calculated based on either ESR or CRP, and DAS28 of more than 5.1 or between 3.2 and 5.1 with radiographic changes was defined as a cut-off point for the initiation of TNF inhibitors. For the maintenance criteria, we used both of improving in DAS28 score (>1.2) and low disease activity (DAS 28<3.2). Differences between the numbers in each step by two criteria were described with Chi-square test and Kappa agreement.
Results
Of the 489 patients in BIOPSY, 299 were included in this study. Among them, 278 patients (93.0%) were eligible of TNF inhibitors when we applied the new initiation criteria with DAS28-ESR, and 244 patients (81.6%) were indicated for TNF inhibitors with DAS28-CRP. For the maintenance criteria, a low disease activity (DAS28<3.2) in 3 months after starting TNF inhibitors is too strict for achieving (33.6% with DAS28-ESR and 50.0% with DAS28-CRP). Instead, decreasing DAS28 by more than 1.2 is more reasonable as a tool for deciding early responsiveness of TNF inhibitors in RA patients (81.2% both with DAS28-ESR and DAS28-CRP).
Conclusion
Our results show that the candidates for TNF inhibitors will be enormously changed according to a change in the reimbursement criteria. To define appropriate patients to receive TNF inhibitors, a further study with regard to the impact of changes in the reimbursement criteria on the outcomes of RA patients will be required.
Go to : 
REFERENCES
1. Abdel-Nasser AM, Rasker JJ, Valkenburg HA. Epidemiological and clinical aspects relating to the variability of rheumatoid arthritis. Semin Arthritis Rheum. 1997; 27:123–40.

3. Sung YK, Cho SK, Choi CB, Bae SC. Prevalence and incidence of rheumatoid arthritis in South Korea. Rheumatol Int. 2013; 33:1525–32.

4. Song JS. Review of tumor necrosis factor inhibitors on rheumatoid arthritis. J Korean Rheum Assoc. 2007; 14:1–14.

5. Gartlehner G, Hansen RA, Jonas BL, Thieda P, Lohr KN. The comparative efficacy and safety of biologics for the treatment of rheumatoid arthritis: a systematic review and metaanalysis. J Rheumatol. 2006; 33:2398–408.
6. Smolen JS, Landewé R, Breedveld FC, Dougados M, Emery P, Gaujoux-Viala C, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis. 2010; 69:964–75.
7. Health Insurance Review & Assessment Service. http://www.hira.or.kr/ebook/0e2acc8a-0c7a-403e-a148-36eb12e7d098/323_Page_img/extra/131001.xml. (accessed 30 NOV 2013).
8. Koike R, Takeuchi T, Eguchi K, Miyasaka N. Japan College of Rheumatology. Update on the Japanese guidelines for the use of infliximab and etanercept in rheumatoid arthritis. Mod Rheumatol. 2007; 17:451–8.

9. Ledingham J, Deighton C. British Society for Rheumatology Standards, Guidelines and Audit Working Group. Update on the British Society for Rheumatology guidelines for prescribing TNFalpha blockers in adults with rheumatoid arthritis (update of previous guidelines of April 2001). Rheumatology (Oxford). 2005; 44:157–63.
10. Son KM, Jung DM, Kim YB, Han JS, Seo YI, Jung YO, et al. Comparison Korean National Health Insurance Reimbursement and other guidelines for TNF-alpha blocker in rheumatoid arthritis. J Rheum Dis. 2012; 19:334–40.
11. Health Insurance Review & Assessment Service. http://www.hira.or.kr/ebook/addf0f38-5003-4c74-9871-6bae668761c9/338_Page_img/extra/140107.xml. (accessed 30 JAN 2014).
12. UMC Nijmegen. DAS score. http://www.das-score.nl./. (ac-cessed 30 Nov. 2013.
13. Inoue E, Yamanaka H, Hara M, Tomatsu T, Kamatani N. Comparison of Disease Activity Score (DAS)28-erythro-cyte sedimentation rate and DAS28-C-reactive protein threshold values. Ann Rheum Dis. 2007; 66:407–9.
14. Castrejón I, Ortiz AM, Garcí a-Vicuña R, Lopez-Bote JP, Humbría A, Carmona L, et al. Are the C-reactive protein values and erythrocyte sedimentation rate equivalent when estimating the 28-joint disease activity score in rheumatoid arthritis? Clin Exp Rheumatol. 2008; 26:769–75.
15. Wells G, Becker JC, Teng J, Dougados M, Schiff M, Smolen J, et al. Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis. 2009; 68:954–60.

16. Matsui T, Kuga Y, Nishino J, Kaneko A, Eto Y, Tohma S. Comparison of composite disease activity indices for rheumatoid arthritis. Mod Rheumatol. 2011; 21:134–43.

17. Matsui T, Kuga Y, Kaneko A, Nishino J, Eto Y, Chiba N, et al. Disease Activity Score 28 (DAS28) using C-reactive protein underestimates disease activity and overestimates EULAR response criteria compared with DAS28 using erythrocyte sedimentation rate in a large observational cohort of rheumatoid arthritis patients in Japan. Ann Rheum Dis. 2007; 66:1221–6.

18. National Health Insurance Administration, Tawan. http://www.nhi.gov.tw/. (accessed 30 Nov. 2013.
19. Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012; 64:625–39.

20. Putrik P, Ramiro S, Kvien TK, Sokka T, Uhlig T, Boonen A. on behalf of Equity in Clinical Eligibility Criteria for RA treatment Working Group. Variations in criteria regulating treatment with reimbursed biologic DMARDs across European countries. Are differences related to country's wealth? Ann Rheum Dis. 2013; [Epub ahead of print].
21. Shaver TS, Anderson JD, Weidensaul DN, Shahouri SH, Busch RE, Mikuls TR, et al. The problem of rheumatoid arthritis disease activity and remission in clinical practice. J Rheumatol. 2008; 35:1015–22.
Go to : 
Table 1.
Baseline characteristics in RA patients using TNF inhibitor for 3 months
Table 2.
Eligibility with the new criteria of RA patients starting TNF inhibitor satisfied with the past initiation criteria (n=299)
|
DAS28-ESR >5.1 or 3.2∼5.1 with radiographic change |
DAS28-CRP >5.1 or 3.2∼5.1 with radiographic change |
||
|---|---|---|---|
| Satisfied | Unsatisfied | Satisfied | Unsatisfied |
| 278 (93.0) | 21 (7.0) | 244 (81.6) | 55 (18.4) |
Table 3.
Distribution change by the maintenance criteria after 3 months in RA patients using TNF inhibitor among patients satisfied with new the initiation criteria
Table 4.
Comparing criteria for eligibility and maintenance of treatment with biological DMARDs in 10 countries
| Country | Requirement to start biological DMARDs | Criteria to maintain biological DMARDs at 6 months* |
|---|---|---|
| Republic of Korea7 | • ESR >28 mm/hr or CRP >2.0 mg/dL | • ESR (≤28 mm/hr or improvement ≥20%) or |
| • Morning stiffness ≥45 min | CRP(≤2.0 mg/dL or improvement ≥20%) | |
| • Active joint count (tender & swollen) ≥20 or 6 (including 4 large joints) | • Active joint count (tender & swollen) ≤50% at 3 months | |
| • DMARDs ≥2 including MTX & 3 months/each DMARD | None | |
| Japan8 | • Active joint count (tender and swollen) ≥6 | |
| • ESR ≥28 mm/hr or CRP ≥2.0 mg/dL | ||
| • 2∼3 months/each DMARD | ||
| Taiwan18 | • 2 DMARDs including MTX | None |
| • DAS28 >3.2 (or improvement ≤1.2) after DMARDs or | ||
| DAS28 >5.1 (for 2 months) | ||
| U.S.A19 | • DMARDs ≥1 for 3 months | • DAS28 <3.2 at 3 months |
| • DAS28 >5.1 | ||
| Denmark20 | • 2 DMARDs: MTX (25 mg/week) and SSZ (2 g/day) | None |
| • DAS28 >3.2 or radiographic progression | ||
| France20 | • None to 1 DMARDs: for the majority of biologics only | None |
| MTX is mentioned without any strict dose or regimen | ||
| • DAS28 >5.1 or lower if corticodependence or structural damage progression | ||
| Germany20 | • No requirement (2 DMARDs failed: including MTX, LEF, | None |
| SSZ, HCQ, Gold and CyA recommended) | ||
| Spain20 | • 1 DMARD: MTX (25 mg/week) | |
| • DAS28 ≥3.2 or SDAI ≥11 or [(DAS28 between 2.6∼3.2 or SDAI 5∼11) and persistent inflammation in joints considered important for the patient that does not resolve with local therapies or significant radiographic progression)] | • DAS28 ≥3.2 or SDAI ≥11 or [(DAS28 between 2.6∼3.2 or SDAI 5∼11) and persistent inflammation in joints considered important for the patient that does not resolve with local therapies or significant radiographic progression)] | |
| Sweden20 | • DAS28 >3.2 and several negative prognostic factors or | • Switch at 6 months if DAS28>3.2 and several |
| DAS28 >5.1 | negative prognostic factors | |
| U.K9 | • 2 DMARDs including MTX | • If improvement in DAS28≥1.2 |
| • DAS28 >5.1 |
* When criteria at different time points or decision were defined, these are also added in the table with the corresponding information.
DMARD: disease modifying antirheumatic drug, ESR: erythrocyte sedimentation rate, CRP: C-reactive protein, MTX: Methotrexate, DAS28: disease activity score with 28-joint assessment, SSZ: sulfasalazine, LEF: leflunomide, HCQ: hydroxychloroquine, CyA: cyclosporine, SDAI: simple disease activity index.
Supplementary Table 1.
Baseline characteristics in RA patients using the criteria with DAS28-ESR
Supplementary Table 2.
Baseline characteristics in RA patients using the criteria with DAS28-CRP




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download