Abstract
A 54-year-old male on chronic hemodialysis, who was taking rifampicin for tuberculous lymphadenitis, was admitted for an acute gout attack. After administrating 3.6 mg of colchicine for 2 days, symptoms began to alleviate. Despite the relatively high dosage in this end-stage renal disease patient, there were no adverse effects, such as diarrhea, vomiting, or myopathy. After 1 and 6 hours of 0.6 mg colchicine administration, serum colchicine was 1.3930 ng/mL and 0.2464 ng/mL, respectively. These values were lower than the mean concentrations in 13 other patients with chronic kidney disease (CKD) after the same time intervals (4.34±0.56 ng/mL and 1.49±0.15 ng/mL, respectively). As rifampicin is an inducer of cytochrome P450 3A4, metabolism of colchicine had increased. When taking colchicine and rifampicin simultaneously, a higher colchicine dose may be needed for the treatment of acute gout in patients with CKD.
Go to : 
REFERENCES
1. Zhang W, Doherty M, Bardin T, Pascual E, Barskova V, Conaghan P, et al. EULAR Standing Committee for International Clinical Studies Including Therapeutics. EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2006; 65:1312–24.

2. United States Food and Drug Administration. Rheumatology information: non-selective non-steroidal anti-inflammatory drugs [internet]. Available from:. http://www.fda.gov/Drugs/ResourcesForYou/HealthProfessionals/ucm106979.htm.
3. Mutual Pharmaceutical Company I. ColcrysⓇ oral tablets: US prescribing information [internet]. Available from. http://www.colcrys.com/assets/pdf/COLCRYS_Full_Prescribing_Information.xml.
4. Terkeltaub RA. Clinical practice. Gout. N Engl J Med. 2003; 349:1647–55.
5. Terkeltaub RA, Furst DE, Bennett K, Kook KA, Crockett RS, Davis MW. High versus low dosing of oral colchicine for early acute gout flare: Twenty-four-hour outcome of the first multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study. Arthritis Rheum. 2010; 62:1060–8.

6. Nuki G. Colchicine: its mechanism of action and efficacy in crystal-induced inflammation. Curr Rheumatol Rep. 2008; 10:218–27.

7. Zhou SF. Drugs behave as substrates, inhibitors and inducers of human cytochrome P450 3A4. Curr Drug Metab. 2008; 9:310–22.

8. Indiana university. Division of clinical pharmacology. P450 Drug interaction table. [internet]. Available from:. http://medicine.iupui.edu/clinpharm/ddis/main-table/.
Go to : 
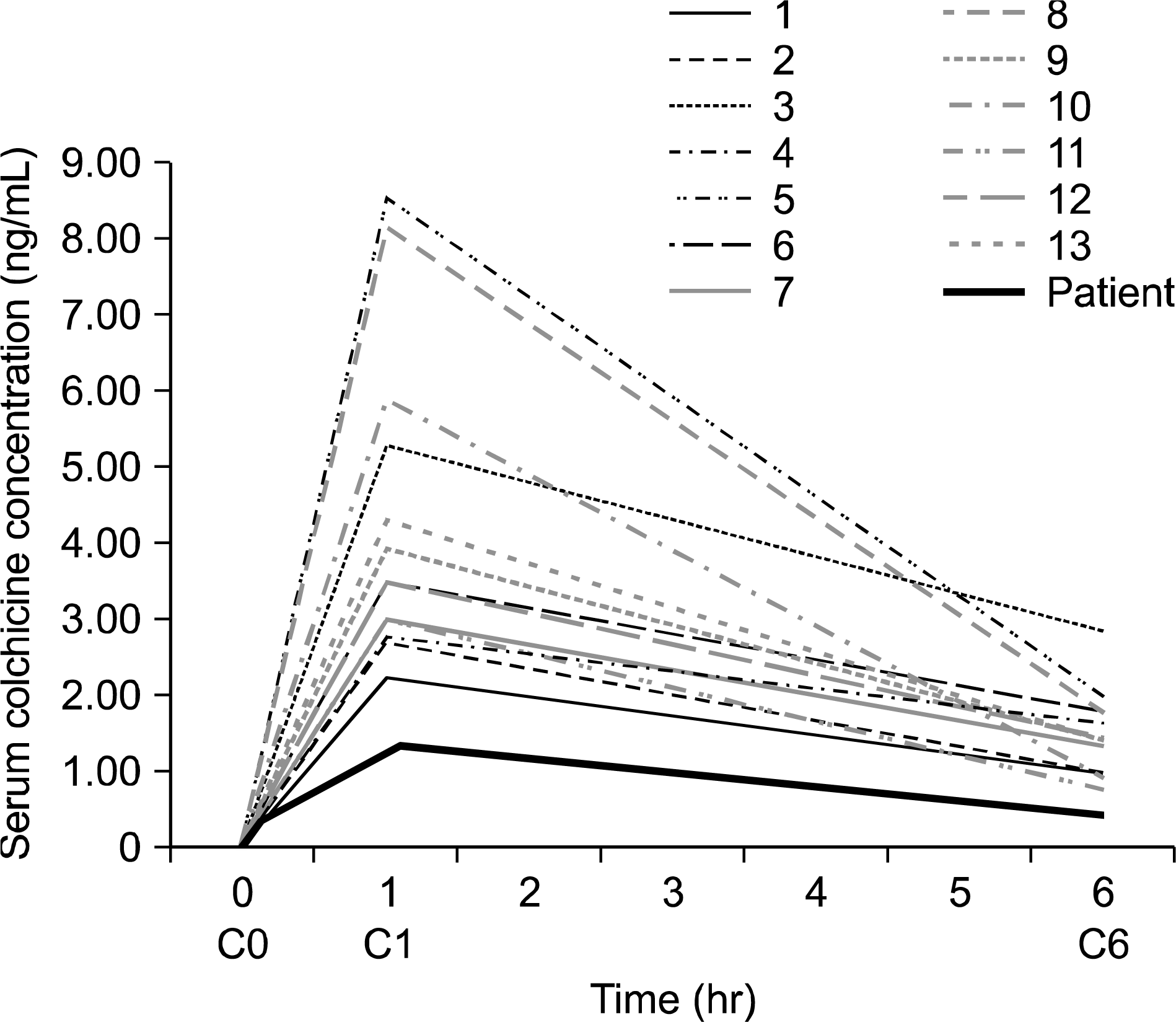 | Figure 1.Time dependent serum level of colchicine. In 13 patients, the serum colchicine concentration curve was drawn according to C0, C1 and C6. We assumed that Tmax was an hour after administration. The lowest line shows the result of the case-study patient. Tmax is the time after administration of a drug when the maximum plasma concentration is reached. |
Table 1.
Laboratory results on day of admission and 1 day before discharge




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download